Comparison of immunogenicity between codon optimized HIV-1 Thailand subtype B gp140 and gp145 vaccines
- PMID: 17350736
- PMCID: PMC1961633
- DOI: 10.1016/j.vaccine.2007.01.118
Comparison of immunogenicity between codon optimized HIV-1 Thailand subtype B gp140 and gp145 vaccines
Abstract
HIV-1 pandemic posed an unprecedented challenge to the global health and it is believed that an effective vaccine will be the final solution against HIV-1. HIV-1 envelope is the primary immunogen in developing neutralization antibody based HIV vaccine. To define the suitable Env derived immunogen, we systemically compared the immunogenicity of gp140 and gp145 in a DNA vaccination alone and a prime-boost modalities. Two DNA vaccines and two recombinant Tiantan vaccinia vaccines (rTTV) were constructed for vaccination of female Balb/c mice. Elispot assay was used to read out the T cell immunity and ELISA assay was used to quantify antibody immunity. PLL (poly-L-leucine)-ELISA assay was used in linear antibody epitope mapping. Mice primed with gp145 tended to elicit more Env-specific T cells responses than those primed with gp140, significant difference was observed in DNA immunization alone. The ultimate T cell responses in prime-boost regimen tend to be determined mainly by the priming efficacy. Linear antibody epitope mapping displayed that sera raised by gp145 priming were vigorously reactive to more peptides than that by gp140. Our data demonstrated HIV-1 Thailand B-derived gp145 may raise higher T-cell responses and broader linear peptide-specific antibody responses than gp140 does. However, it remains to be determined that how these observations are relevant to the neutralization of antibody activities.
Figures
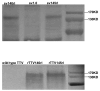

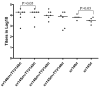
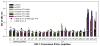







Similar articles
-
Broad HIV-1 neutralizing antibody response induced by heterologous gp140/gp145 DNA prime-vaccinia boost immunization.Vaccine. 2012 Jun 13;30(28):4135-43. doi: 10.1016/j.vaccine.2012.04.075. Epub 2012 May 2. Vaccine. 2012. PMID: 22561314 Free PMC article.
-
Immunogenicity comparison between codon optimized HIV-1 CRF BC_07 gp140 and gp145 vaccines.AIDS Res Hum Retroviruses. 2007 Nov;23(11):1396-404. doi: 10.1089/aid.2007.0131. AIDS Res Hum Retroviruses. 2007. PMID: 18184083
-
Deglycosylation or partial removal of HIV-1 CN54 gp140 V1/V2 domain enhances env-specific T cells.AIDS Res Hum Retroviruses. 2009 Jun;25(6):607-17. doi: 10.1089/aid.2008.0289. AIDS Res Hum Retroviruses. 2009. PMID: 19500018
-
Immunogenicity of DNA vaccines in humans: it takes two to tango.Hum Vaccin. 2008 Nov-Dec;4(6):449-52. doi: 10.4161/hv.4.6.6179. Epub 2008 Nov 28. Hum Vaccin. 2008. PMID: 18443427 Review.
-
Innovative bioinformatic approaches for developing peptide-based vaccines against hypervariable viruses.Immunol Cell Biol. 2011 Jan;89(1):81-9. doi: 10.1038/icb.2010.65. Epub 2010 May 11. Immunol Cell Biol. 2011. PMID: 20458336 Review.
Cited by
-
Broad HIV-1 neutralizing antibody response induced by heterologous gp140/gp145 DNA prime-vaccinia boost immunization.Vaccine. 2012 Jun 13;30(28):4135-43. doi: 10.1016/j.vaccine.2012.04.075. Epub 2012 May 2. Vaccine. 2012. PMID: 22561314 Free PMC article.
-
Boosting functional avidity of CD8+ T cells by vaccinia virus vaccination depends on intrinsic T-cell MyD88 expression but not the inflammatory milieu.J Virol. 2014 May;88(10):5356-68. doi: 10.1128/JVI.03664-13. Epub 2014 Feb 19. J Virol. 2014. PMID: 24554667 Free PMC article.
-
A novel high-throughput vaccinia virus neutralization assay and preexisting immunity in populations from different geographic regions in China.PLoS One. 2012;7(3):e33392. doi: 10.1371/journal.pone.0033392. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438922 Free PMC article.
-
Neutralizing antibodies against adenovirus type 2 in normal and HIV-1-infected subjects: Implications for use of Ad2 vectors in vaccines.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-8. doi: 10.1080/21645515.2017.1281487. Epub 2017 Mar 16. Hum Vaccin Immunother. 2017. PMID: 28301274 Free PMC article.
References
-
- Pitisuttithum P, Berman PW, Phonrat B, Suntharasamai P, Raktham S, et al. PhaseI/II study of a candidate vaccine designed against the B and E subtypes of HIV-1. J AIDS. 2004;37:1160–65. - PubMed
-
- Desrosiers RC. Prospects for an AIDS vaccine. Nat Med. 2004;10:221–23. - PubMed
-
- Gotch FM, Imami N, Hardy G. Candidate vaccines for immunotherapy in HIV. HIV Med. 2001;2:260–265. - PubMed
-
- Ross RW, Wright ME, Tavel JA. Ongoing trials of immunebased therapies for HIV infection in adults. Exp Opin Biol Ther. 2001;1:413–424. - PubMed
-
- Girard M, Habel A, Chanel C. New prospects for the development of a vaccine against human immunodeficiency virus type 1: an overview. C R Acad Sci. 1999;III 322:959–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

