Uninfected mosquito bites confer protection against infection with malaria parasites
- PMID: 17339356
- PMCID: PMC1865743
- DOI: 10.1128/IAI.01928-06
Uninfected mosquito bites confer protection against infection with malaria parasites
Abstract
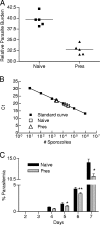
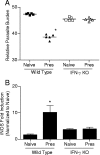
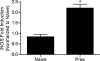
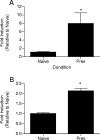
Despite decades of research and multiple initiatives, malaria continues to be one of the world's most debilitating infectious diseases. New insights for malaria control and vaccine development will be essential to thwart the staggering worldwide impact of this disease (A. Bjorkman and A. Bhattarai, Acta Trop. 94:163-169, 2005); ultimately successful vaccine strategies will undoubtedly be multifactorial, incorporating multiple antigens and targeting diverse aspects of the malaria parasites' biology (M. F. Good et al., Immunol. Rev. 201:254-267, 2004). Using a murine model of malaria infection, we show here that exposure to bites from uninfected mosquitoes prior to Plasmodium yoelii infection influences the local and systemic immune responses and limits parasite development within the host. In hosts preexposed to bites from uninfected mosquitoes, reduced parasite burdens in the livers were detected early, and during the blood-stage of the life cycle, these burdens remained lower than those in hosts that received mosquito bites only at the time of infection. Repeated exposure to bites from uninfected mosquitoes skewed the immune response towards a T-helper 1 (Th1) phenotype as indicated by increased levels of interleukin-12, gamma interferon, and inducible nitric oxide synthase. These data suggest that the addition of mosquito salivary components to antimalaria vaccines may be a viable strategy for creating a Th1-biased environment known to be effective against malaria infection. Furthermore, this strategy may be important for the development of vaccines to combat other mosquito-transmitted pathogens.
Figures

Similar articles
-
Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells.J Infect Dis. 2007 Aug 15;196(4):608-16. doi: 10.1086/519742. Epub 2007 Jul 9. J Infect Dis. 2007. PMID: 17624848
-
Midgut specific immune response of vector mosquito Anopheles stephensi to malaria parasite Plasmodium.Indian J Exp Biol. 2001 Mar;39(3):287-90. Indian J Exp Biol. 2001. PMID: 11495292
-
Genetically attenuated parasite vaccines induce contact-dependent CD8+ T cell killing of Plasmodium yoelii liver stage-infected hepatocytes.J Immunol. 2009 Nov 1;183(9):5870-8. doi: 10.4049/jimmunol.0900302. Epub 2009 Oct 7. J Immunol. 2009. PMID: 19812194
-
Immune interactions between mosquitoes and their hosts.Parasite Immunol. 2006 Apr;28(4):143-53. doi: 10.1111/j.1365-3024.2006.00805.x. Parasite Immunol. 2006. PMID: 16542316 Review.
-
[A new view of malaria provided by parasite imaging].Bull Acad Natl Med. 2007 Oct;191(7):1261-70; discussion 1271. Bull Acad Natl Med. 2007. PMID: 18447048 Review. French.
Cited by
-
Neither mosquito saliva nor immunity to saliva has a detectable effect on the infectivity of Plasmodium sporozoites injected into mice.Infect Immun. 2010 Jan;78(1):545-51. doi: 10.1128/IAI.00807-09. Epub 2009 Nov 2. Infect Immun. 2010. PMID: 19884338 Free PMC article.
-
MicroRNAs and other small RNAs in Aedes aegypti saliva and salivary glands following chikungunya virus infection.Sci Rep. 2022 Jun 9;12(1):9536. doi: 10.1038/s41598-022-13780-3. Sci Rep. 2022. PMID: 35681077 Free PMC article.
-
Differential expression of salivary proteins between susceptible and insecticide-resistant mosquitoes of Culex quinquefasciatus.PLoS One. 2011 Mar 23;6(3):e17496. doi: 10.1371/journal.pone.0017496. PLoS One. 2011. PMID: 21448269 Free PMC article.
-
Vertebrate Responses against Arthropod Salivary Proteins and Their Therapeutic Potential.Vaccines (Basel). 2021 Apr 5;9(4):347. doi: 10.3390/vaccines9040347. Vaccines (Basel). 2021. PMID: 33916367 Free PMC article. Review.
-
Visualizing non infectious and infectious Anopheles gambiae blood feedings in naive and saliva-immunized mice.PLoS One. 2012;7(12):e50464. doi: 10.1371/journal.pone.0050464. Epub 2012 Dec 13. PLoS One. 2012. PMID: 23272060 Free PMC article.
References
-
- Alger, N. E., and J. Harant. 1976. Plasmodium berghei: sporozoite challenge, protection, and hypersensitivity in mice. Exp. Parasitol. 40:273-280. - PubMed
-
- Alger, N. E., J. A. Harant, L. C. Willis, and G. M. Jorgensen. 1972. Sporozoite and normal salivary gland induced immunity in malaria. Nature 238:341. - PubMed
-
- Allen, J. R. 1989. Immunology of interactions between ticks and laboratory animals. Exp. Appl. Acarol. 7:5-13. - PubMed
-
- Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. - PMC - PubMed
-
- Belnoue, E., F. T. Costa, T. Frankenberg, A. M. Vigario, T. Voza, N. Leroy, M. M. Rodrigues, I. Landau, G. Snounou, and L. Renia. 2004. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J. Immunol. 172:2487-2495. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

