Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds
- PMID: 17336390
- PMCID: PMC1905848
- DOI: 10.1016/j.pharmthera.2007.01.006
Structure-nongenomic neuroprotection relationship of estrogens and estrogen-derived compounds
Abstract
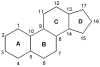
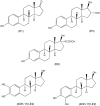
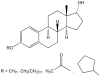
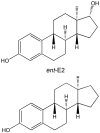
Nongenomic estrogen signaling pathways involve extranuclear estrogen receptors or function independently from estrogen receptors. These pathways participate in neuroprotection elicited by the hormone. Additional nongenomic neuroprotective effects are attributable to antioxidant and antiinflammatory actions of estrogens. Numerous chemical modifications to afford neuroprotective compounds from estrogens while eliminating estrogenicity and maintaining or enhancing nongenomic neuroprotection have been described. This review highlights recent structure-activity studies that revealed the importance of antioxidant effects for neuroprotective estrogen analogues and derivatives.
Figures

Similar articles
-
Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer's disease.BMC Neurosci. 2006 Mar 13;7:24. doi: 10.1186/1471-2202-7-24. BMC Neurosci. 2006. PMID: 16533397 Free PMC article.
-
Neuroprotection against oxidative stress by estrogens: structure-activity relationship.Mol Pharmacol. 1997 Apr;51(4):535-41. Mol Pharmacol. 1997. PMID: 9106616
-
A novel mechanism of non-feminizing estrogens in neuroprotection.Exp Gerontol. 2017 Aug;94:99-102. doi: 10.1016/j.exger.2016.10.013. Epub 2016 Nov 3. Exp Gerontol. 2017. PMID: 27818250 Free PMC article. Review.
-
Oestrogen as a neuroprotective hormone.Nat Rev Neurosci. 2002 Jun;3(6):433-42. doi: 10.1038/nrn846. Nat Rev Neurosci. 2002. PMID: 12042878 Review.
-
From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens.Pharmacol Res. 2005 Aug;52(2):119-32. doi: 10.1016/j.phrs.2005.03.002. Pharmacol Res. 2005. PMID: 15967377 Review.
Cited by
-
Androgens selectively protect against apoptosis in hippocampal neurones.J Neuroendocrinol. 2010 Sep;22(9):1013-22. doi: 10.1111/j.1365-2826.2010.02044.x. Epub 2010 Jun 16. J Neuroendocrinol. 2010. PMID: 20561156 Free PMC article.
-
Estrogens and neuroprotection in retinal diseases.Mol Vis. 2008 Aug 11;14:1480-6. Mol Vis. 2008. Retraction in: Mol Vis. 2008;14:2204. PMID: 18698377 Free PMC article. Retracted. Review.
-
Brain-body mechanisms contribute to sexual dimorphism in amyotrophic lateral sclerosis.Nat Rev Neurol. 2024 Aug;20(8):475-494. doi: 10.1038/s41582-024-00991-7. Epub 2024 Jul 4. Nat Rev Neurol. 2024. PMID: 38965379 Review.
-
Role of protein phosphatases and mitochondria in the neuroprotective effects of estrogens.Front Neuroendocrinol. 2009 Jul;30(2):93-105. doi: 10.1016/j.yfrne.2009.04.013. Epub 2009 May 3. Front Neuroendocrinol. 2009. PMID: 19410596 Free PMC article. Review.
-
Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats.Brain Res. 2008 Jul 7;1218:1-12. doi: 10.1016/j.brainres.2008.04.055. Epub 2008 Apr 30. Brain Res. 2008. PMID: 18513705 Free PMC article.
References
-
- Aloisi AM. Gonadal hormones and sex differences in pain reactivity. Clin J Pain. 2003;19:168–174. - PubMed
-
- Amantea D, Russo R, Bagetta G, Corasaniti MT. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52:119–132. - PubMed
-
- Anstead GM, Carlson KE, Katzenellenbogen JA. The estradiol pharmacophore: Ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids. 1997;62:268–303. - PubMed
-
- Badeau M, Adlercreutz H, Kaihovaara P, Tikkanen M. Estrogen A-ring structure and antioxidative effect on lipoproteins. J Steroid Biochem Mol Biol. 2005;96:271–278. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG010485-080005/AG/NIA NIH HHS/United States
- P01 AG022550-02/AG/NIA NIH HHS/United States
- P01 AG010485-110005/AG/NIA NIH HHS/United States
- P01 AG010485-150005/AG/NIA NIH HHS/United States
- R01 NS044765-05/NS/NINDS NIH HHS/United States
- P01 AG022550/AG/NIA NIH HHS/United States
- P01 AG010485-100005/AG/NIA NIH HHS/United States
- P01 AG010485-070005/AG/NIA NIH HHS/United States
- P01 AG022550-03/AG/NIA NIH HHS/United States
- R01 NS044765/NS/NINDS NIH HHS/United States
- P01 AG010485-09/AG/NIA NIH HHS/United States
- P01 AG010485-120005/AG/NIA NIH HHS/United States
- AG010485/AG/NIA NIH HHS/United States
- AG022550/AG/NIA NIH HHS/United States
- R01 NS044765-02/NS/NINDS NIH HHS/United States
- P01 AG010485/AG/NIA NIH HHS/United States
- R01 NS044765-01/NS/NINDS NIH HHS/United States
- P01 AG010485-14/AG/NIA NIH HHS/United States
- P01 AG010485-090005/AG/NIA NIH HHS/United States
- R01 NS044765-03/NS/NINDS NIH HHS/United States
- P01 AG010485-15/AG/NIA NIH HHS/United States
- NS044765/NS/NINDS NIH HHS/United States
- R01 NS044765-04/NS/NINDS NIH HHS/United States
- P01 AG010485-140005/AG/NIA NIH HHS/United States
- P01 AG022550-04/AG/NIA NIH HHS/United States
- P01 AG022550-01/AG/NIA NIH HHS/United States
- P01 AG010485-14S1/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources

