Mechanoregulation of gene expression in fibroblasts
- PMID: 17331678
- PMCID: PMC2893340
- DOI: 10.1016/j.gene.2007.01.014
Mechanoregulation of gene expression in fibroblasts
Abstract
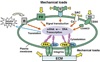
Mechanical loads placed on connective tissues alter gene expression in fibroblasts through mechanotransduction mechanisms by which cells convert mechanical signals into cellular biological events, such as gene expression of extracellular matrix components (e.g., collagen). This mechanical regulation of ECM gene expression affords maintenance of connective tissue homeostasis. However, mechanical loads can also interfere with homeostatic cellular gene expression and consequently cause the pathogenesis of connective tissue diseases such as tendinopathy and osteoarthritis. Therefore, the regulation of gene expression by mechanical loads is closely related to connective tissue physiology and pathology. This article reviews the effects of various mechanical loading conditions on gene regulation in fibroblasts and discusses several mechanotransduction mechanisms. Future research directions in mechanoregulation of gene expression are also suggested.
Figures

Similar articles
-
Gene regulation by mechanotransduction in fibroblasts.Appl Physiol Nutr Metab. 2007 Oct;32(5):967-73. doi: 10.1139/H07-053. Appl Physiol Nutr Metab. 2007. PMID: 18059623 Review.
-
From mechanotransduction to extracellular matrix gene expression in fibroblasts.Biochim Biophys Acta. 2009 May;1793(5):911-20. doi: 10.1016/j.bbamcr.2009.01.012. Epub 2009 Jan 31. Biochim Biophys Acta. 2009. PMID: 19339214 Review.
-
Mechanotransduction and extracellular matrix homeostasis.Nat Rev Mol Cell Biol. 2014 Dec;15(12):802-12. doi: 10.1038/nrm3896. Epub 2014 Oct 22. Nat Rev Mol Cell Biol. 2014. PMID: 25355505 Free PMC article. Review.
-
Mechanical signals regulating extracellular matrix gene expression in fibroblasts.Scand J Med Sci Sports. 2005 Aug;15(4):223-30. doi: 10.1111/j.1600-0838.2005.00461.x. Scand J Med Sci Sports. 2005. PMID: 15998339
-
How do fibroblasts translate mechanical signals into changes in extracellular matrix production?Matrix Biol. 2003 Mar;22(1):73-80. doi: 10.1016/s0945-053x(03)00004-0. Matrix Biol. 2003. PMID: 12714044 Review.
Cited by
-
Why osteoarthritis of the knee is called "a wound that does not heal" and why Tai Chi is an effective treatment.Front Med (Lausanne). 2023 Nov 27;10:1208326. doi: 10.3389/fmed.2023.1208326. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38089871 Free PMC article. Review.
-
Cytoskeletal disassembly and cell rounding promotes adipogenesis from ES cells.Stem Cell Rev Rep. 2010 Mar;6(1):74-85. doi: 10.1007/s12015-010-9115-8. Stem Cell Rev Rep. 2010. PMID: 20148318
-
Identifying the predictors of needling after XEN gel implant.Eye (Lond). 2019 Mar;33(3):353-357. doi: 10.1038/s41433-018-0206-0. Epub 2018 Sep 11. Eye (Lond). 2019. PMID: 30206416 Free PMC article.
-
Altered Mesenchymal Stem Cells Mechanotransduction from Oxidized Collagen: Morphological and Biophysical Observations.Int J Mol Sci. 2023 Feb 11;24(4):3635. doi: 10.3390/ijms24043635. Int J Mol Sci. 2023. PMID: 36835046 Free PMC article.
-
Animal Models for Studies of Keloid Scarring.Adv Wound Care (New Rochelle). 2019 Feb 1;8(2):77-89. doi: 10.1089/wound.2018.0828. Epub 2019 Feb 13. Adv Wound Care (New Rochelle). 2019. PMID: 31832272 Free PMC article. Review.
References
-
- Abrahams M. Mechanical behaviour of tendon in vitro. A preliminary report. Med. Biol. Eng. 1967;5:433–443. - PubMed
-
- Almekinders LC, Banes AJ, Ballenger CA. Effects of repetitive motion on human fibroblasts. Med. Sci. Sports Exerc. 1993;25:603–607. - PubMed
-
- Amano M, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
-
- Amano M, et al. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
-
- Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J. Orthop. Res. 2002a;20:36–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

