Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques
- PMID: 17329330
- PMCID: PMC1900241
- DOI: 10.1128/JVI.00055-07
Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques
Abstract
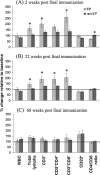
Since human immunodeficiency virus (HIV)-specific cell-mediated immune (CMI) responses are critical in the early control and resolution of HIV infection and correlate with postchallenge outcomes in rhesus macaque challenge experiments, we sought to identify a plasmid DNA (pDNA) vaccine design capable of eliciting robust and balanced CMI responses to multiple HIV type 1 (HIV-1)-derived antigens for further development. Previously, a number of two-, three-, and four-vector pDNA vaccine designs were identified as capable of eliciting HIV-1 antigen-specific CMI responses in mice (M. A. Egan et al., Vaccine 24:4510-4523, 2006). We then sought to further characterize the relative immunogenicities of these two-, three-, and four-vector pDNA vaccine designs in nonhuman primates and to determine the extent to which in vivo electroporation (EP) could improve the resulting immune responses. The results indicated that a two-vector pDNA vaccine design elicited the most robust and balanced CMI response. In addition, vaccination in combination with in vivo EP led to a more rapid onset and enhanced vaccine-specific immune responses. In macaques immunized in combination with in vivo EP, we observed a 10- to 40-fold increase in HIV-specific enzyme-linked immunospot assay responses compared to those for macaques receiving a 5-fold higher dose of vaccine without in vivo EP. This increase in CMI responses translates to an apparent 50- to 200-fold increase in pDNA vaccine potency. Importantly, in vivo EP enhanced the immune response against the less immunogenic antigens, resulting in a more balanced immune response. In addition, in vivo EP resulted in an approximate 2.5-log(10) increase in antibody responses. The results further indicated that in vivo EP was associated with a significant reduction in pDNA persistence and did not result in an increase in pDNA associated with high-molecular-weight DNA relative to macaques receiving the pDNA without EP. Collectively, these results have important implications for the design and development of an efficacious vaccine for the prevention of HIV-1 infection.
Figures


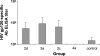
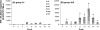
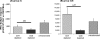
Similar articles
-
Modifying the HIV-1 env gp160 gene to improve pDNA vaccine-elicited cell-mediated immune responses.Vaccine. 2008 Sep 19;26(40):5083-94. doi: 10.1016/j.vaccine.2008.03.092. Epub 2008 Apr 24. Vaccine. 2008. PMID: 18485543 Free PMC article.
-
Rational design of a plasmid DNA vaccine capable of eliciting cell-mediated immune responses to multiple HIV antigens in mice.Vaccine. 2006 May 22;24(21):4510-23. doi: 10.1016/j.vaccine.2005.08.024. Epub 2005 Aug 19. Vaccine. 2006. PMID: 16140439
-
Combined effects of IL-12 and electroporation enhances the potency of DNA vaccination in macaques.Vaccine. 2008 Jun 13;26(25):3112-20. doi: 10.1016/j.vaccine.2008.02.036. Epub 2008 Mar 11. Vaccine. 2008. PMID: 18430495 Free PMC article.
-
DNA vaccines against human immunodeficiency virus type 1 in the past decade.Clin Microbiol Rev. 2004 Apr;17(2):370-89. doi: 10.1128/CMR.17.2.370-389.2004. Clin Microbiol Rev. 2004. PMID: 15084506 Free PMC article. Review.
-
Electroporation delivery of DNA vaccines: prospects for success.Curr Opin Immunol. 2011 Jun;23(3):421-9. doi: 10.1016/j.coi.2011.03.008. Epub 2011 Apr 27. Curr Opin Immunol. 2011. PMID: 21530212 Free PMC article. Review.
Cited by
-
Safety and immunogenicity of an HIV-1 gag DNA vaccine with or without IL-12 and/or IL-15 plasmid cytokine adjuvant in healthy, HIV-1 uninfected adults.PLoS One. 2012;7(1):e29231. doi: 10.1371/journal.pone.0029231. Epub 2012 Jan 5. PLoS One. 2012. PMID: 22242162 Free PMC article. Clinical Trial.
-
Biodistribution, persistence and lack of integration of a multigene HIV vaccine delivered by needle-free intradermal injection and electroporation.Vaccine. 2010 Nov 29;28(51):8203-9. doi: 10.1016/j.vaccine.2010.08.108. Epub 2010 Oct 15. Vaccine. 2010. PMID: 20951666 Free PMC article.
-
Markedly enhanced immunogenicity of a Pfs25 DNA-based malaria transmission-blocking vaccine by in vivo electroporation.Vaccine. 2008 Jan 10;26(2):185-92. doi: 10.1016/j.vaccine.2007.10.066. Epub 2007 Nov 20. Vaccine. 2008. PMID: 18054817 Free PMC article.
-
Inflammasomes in antiviral immunity: clues for influenza vaccine development.Clin Exp Vaccine Res. 2014 Jan;3(1):5-11. doi: 10.7774/cevr.2014.3.1.5. Epub 2013 Dec 18. Clin Exp Vaccine Res. 2014. PMID: 24427758 Free PMC article. Review.
-
SIV antigen-specific effects on immune responses induced by vaccination with DNA electroporation and plasmid IL-12.Vaccine. 2013 Oct 1;31(42):4749-58. doi: 10.1016/j.vaccine.2013.08.011. Epub 2013 Aug 14. Vaccine. 2013. PMID: 23954384 Free PMC article.
References
-
- Aihara, H., and J. Miyazaki. 1998. Gene transfer into muscle by electroporation in vivo. Nat. Biotechnol. 16:867-870. - PubMed
-
- Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. - PubMed
-
- Babiuk, L. A., S. van Drunen Littel-van den Hurk, and S. L. Babiuk. 1999. Immunization of animals: from DNA to the dinner plate. Vet. Immunol. Immunopathol. 72:189-202. - PubMed
-
- Babiuk, S., M. E. Baca-Estrada, M. Foldvari, M. Storms, D. Rabussay, G. Widera, and L. A. Babiuk. 2002. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine 20:3399-3408. - PubMed
-
- Bachy, M., F. Boudet, M. Bureau, Y. Girerd-Chambaz, P. Wils, D. Scherman, and C. Meric. 2001. Electric pulses increase the immunogenicity of an influenza DNA vaccine injected intramuscularly in the mouse. Vaccine 19:1688-1693. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

