Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice
- PMID: 17322373
- PMCID: PMC1864882
- DOI: 10.2353/ajpath.2007.060816
Murine FLIP transgene expressed on thyroid epithelial cells promotes resolution of granulomatous experimental autoimmune thyroiditis in DBA/1 mice
Abstract
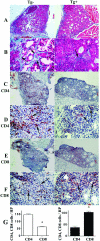
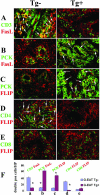
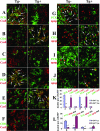
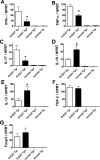
Granulomatous experimental autoimmune thyroiditis (G-EAT) is induced by mouse thyroglobulin-sensitized splenocytes activated in vitro with mouse thyroglobulin and interleukin-12. In wild-type (WT) DBA/1 recipients of WT donor splenocytes, thyroid lesions reach maximal severity at day 20, with ongoing inflammation and extensive fibrosis at day 60. Our previous studies indicated the site of expression of FLIP and Fas ligand [thyroid epithelial cells (TECs) versus inflammatory cells] differed in mice when lesions would resolve or progress to fibrosis. To test the hypothesis that expression of FLIP by TECs would promote earlier G-EAT resolution in DBA/1 mice, transgenic (Tg) DBA/1 mice expressing FLIP on TECs were generated. In FLIP Tg(+) and Tg(-) littermate recipients of WT donor splenocytes, G-EAT severity was comparable at day 20, but fibrosis was decreased, and many lesions resolved by day 60 in Tg(+) but not Tg(-) recipients. FLIP and Fas ligand were primarily expressed by TECs in Tg(+) recipients and by inflammatory cells in Tg(-) recipients at day 60. Apoptosis of inflammatory cells was greater, and expression of proinflammatory cytokines was decreased in thyroids of Tg(+) compared with Tg(-) recipients. These results are consistent with the hypothesis that transgenic expression of FLIP on thyroid epithelial cells promotes earlier resolution of granulomatous experimental autoimmune thyroiditis.
Figures

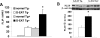
Similar articles
-
Expression of transgenic FLIP on thyroid epithelial cells inhibits induction and promotes resolution of granulomatous experimental autoimmune thyroiditis in CBA/J mice.Endocrinology. 2007 Dec;148(12):5734-45. doi: 10.1210/en.2007-0939. Epub 2007 Sep 6. Endocrinology. 2007. PMID: 17823262
-
Fas ligand is required for resolution of granulomatous experimental autoimmune thyroiditis.J Immunol. 2004 Dec 15;173(12):7615-21. doi: 10.4049/jimmunol.173.12.7615. J Immunol. 2004. PMID: 15585889
-
Cultured murine thyroid epithelial cells expressing transgenic Fas-associated death domain-like interleukin-1beta converting enzyme inhibitory protein are protected from fas-mediated apoptosis.Endocrinology. 2008 Jul;149(7):3321-9. doi: 10.1210/en.2008-0080. Epub 2008 Mar 20. Endocrinology. 2008. PMID: 18356280 Free PMC article.
-
Adoptive transfer murine model of granulomatous experimental autoimmune thyroiditis.Int Rev Immunol. 2000;19(6):535-55. doi: 10.3109/08830180009088511. Int Rev Immunol. 2000. PMID: 11129114 Review.
-
Molecular parameters linking thyroglobulin iodination with autoimmune thyroiditis.Hormones (Athens). 2011 Jan-Mar;10(1):27-35. doi: 10.14310/horm.2002.1290. Hormones (Athens). 2011. PMID: 21349803 Review.
Cited by
-
Resveratrol enhances radiation sensitivity in prostate cancer by inhibiting cell proliferation and promoting cell senescence and apoptosis.Cancer Sci. 2012 Jun;103(6):1090-8. doi: 10.1111/j.1349-7006.2012.02272.x. Epub 2012 Apr 15. Cancer Sci. 2012. PMID: 22417066 Free PMC article.
-
Apoptosis modulation as a promising target for treatment of systemic sclerosis.Int J Rheumatol. 2011;2011:495792. doi: 10.1155/2011/495792. Epub 2011 Sep 6. Int J Rheumatol. 2011. PMID: 21912551 Free PMC article.
-
Loss of monocyte chemoattractant protein-1 alters macrophage polarization and reduces NFκB activation in the foreign body response.Acta Biomater. 2015 Jan;11:37-47. doi: 10.1016/j.actbio.2014.09.022. Epub 2014 Sep 19. Acta Biomater. 2015. PMID: 25242651 Free PMC article.
-
TGF-β promotes proliferation of thyroid epithelial cells in IFN-γ(-/-) mice by down-regulation of p21 and p27 via AKT pathway.Am J Pathol. 2012 Feb;180(2):650-60. doi: 10.1016/j.ajpath.2011.10.009. Epub 2011 Nov 24. Am J Pathol. 2012. PMID: 22119715 Free PMC article.
-
Trichomonas vaginalis: a possible foe to prostate cancer.Med Oncol. 2016 Oct;33(10):115. doi: 10.1007/s12032-016-0832-y. Epub 2016 Sep 8. Med Oncol. 2016. PMID: 27613161
References
-
- Lira SA, Martin AP, Marinkovic T, Furtado GC. Mechanisms regulating lymphocytic infiltration of the thyroid in murine models of thyroiditis. Crit Rev Immunol. 2005;25:251–262. - PubMed
-
- Braley-Mullen H, Johnson M, Sharp GC, Kyriakos M. Induction of experimental autoimmune thyroiditis in mice with in vitro activated splenic T cells. Cell Immunol. 1985;93:132–143. - PubMed
-
- Conaway DH, Giraldo AA, David CS, Kong YC. In situ analysis of T cell subset composition in experimental autoimmune thyroiditis after adoptive transfer of activated spleen cells. Cell Immunol. 1990;125:247–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

