De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated
- PMID: 17322335
- PMCID: PMC1851831
- DOI: 10.1104/pp.106.094110
De novo formation of plant endoplasmic reticulum export sites is membrane cargo induced and signal mediated
Abstract
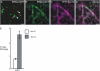
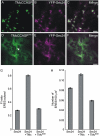
The plant endoplasmic reticulum (ER) contains functionally distinct subdomains at which cargo molecules are packed into transport carriers. To study these ER export sites (ERES), we used tobacco (Nicotiana tabacum) leaf epidermis as a model system and tested whether increased cargo dosage leads to their de novo formation. We have followed the subcellular distribution of the known ERES marker based on a yellow fluorescent protein (YFP) fusion of the Sec24 COPII coat component (YFP-Sec24), which, differently from the previously described ERES marker, tobacco Sar1-YFP, is visibly recruited at ERES in both the presence and absence of overexpressed membrane cargo. This allowed us to quantify variation in the ERES number and in the recruitment of Sec24 to ERES upon expression of cargo. We show that increased synthesis of membrane cargo leads to an increase in the number of ERES and induces the recruitment of Sec24 to these ER subdomains. Soluble proteins that are passively secreted were found to leave the ER with no apparent up-regulation of either the ERES number or the COPII marker, showing that bulk flow transport has spare capacity in vivo. However, de novo ERES formation, as well as increased recruitment of Sec24 to ERES, was found to be dependent on the presence of the diacidic ER export motif in the cytosolic domain of the membrane cargo. Our data suggest that the plant ER can adapt to a sudden increase in membrane cargo-stimulated secretory activity by signal-mediated recruitment of COPII machinery onto existing ERES, accompanied by de novo generation of new ERES.
Figures
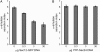

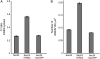
Similar articles
-
Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites.Plant Cell. 2005 May;17(5):1513-31. doi: 10.1105/tpc.104.026757. Epub 2005 Apr 1. Plant Cell. 2005. PMID: 15805489 Free PMC article.
-
Endoplasmic reticulum export sites and Golgi bodies behave as single mobile secretory units in plant cells.Plant Cell. 2004 Jul;16(7):1753-71. doi: 10.1105/tpc.022673. Epub 2004 Jun 18. Plant Cell. 2004. PMID: 15208385 Free PMC article.
-
Dynamic organization of COPII coat proteins at endoplasmic reticulum export sites in plant cells.Plant J. 2009 Mar;57(6):963-74. doi: 10.1111/j.1365-313X.2008.03740.x. Epub 2008 Dec 4. Plant J. 2009. PMID: 19000162
-
The ER exit sites are specialized ER zones for the transport of cargo proteins from the ER to the Golgi apparatus.J Biochem. 2019 Feb 1;165(2):109-114. doi: 10.1093/jb/mvy080. J Biochem. 2019. PMID: 30304445 Review.
-
A systematic review of Sec24 cargo interactome.Traffic. 2021 Dec;22(12):412-424. doi: 10.1111/tra.12817. Epub 2021 Oct 5. Traffic. 2021. PMID: 34533884 Review.
Cited by
-
ER-associated VAP27-1 and VAP27-3 proteins functionally link the lipid-binding ORP2A at the ER-chloroplast contact sites.Nat Commun. 2024 Jul 17;15(1):6008. doi: 10.1038/s41467-024-50425-7. Nat Commun. 2024. PMID: 39019917 Free PMC article.
-
Adaptation of endoplasmic reticulum exit sites to acute and chronic increases in cargo load.EMBO J. 2008 Aug 6;27(15):2043-54. doi: 10.1038/emboj.2008.136. Epub 2008 Jul 24. EMBO J. 2008. PMID: 18650939 Free PMC article.
-
Advances in fluorescent protein-based imaging for the analysis of plant endomembranes.Plant Physiol. 2008 Aug;147(4):1469-81. doi: 10.1104/pp.108.120147. Plant Physiol. 2008. PMID: 18678739 Free PMC article. Review. No abstract available.
-
Links between lipid homeostasis, organelle morphodynamics and protein trafficking in eukaryotic and plant secretory pathways.Plant Cell Rep. 2011 Feb;30(2):177-93. doi: 10.1007/s00299-010-0954-1. Epub 2010 Dec 1. Plant Cell Rep. 2011. PMID: 21120657 Review.
-
Redundant function of two Arabidopsis COPII components, AtSec24B and AtSec24C, is essential for male and female gametogenesis.Planta. 2013 Sep;238(3):561-75. doi: 10.1007/s00425-013-1913-1. Epub 2013 Jun 19. Planta. 2013. PMID: 23779001
References
-
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3 531–537 - PubMed
-
- Barlowe C (2003) Signals for COPII-dependent export from the ER: what's the ticket out? Trends Cell Biol 13 295–300 - PubMed
-
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77 895–907 - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

