Anti HIV-1 virucidal activity of polyamide nucleic acid-membrane transducing peptide conjugates targeted to primer binding site of HIV-1 genome
- PMID: 17320140
- PMCID: PMC2038983
- DOI: 10.1016/j.virol.2007.01.016
Anti HIV-1 virucidal activity of polyamide nucleic acid-membrane transducing peptide conjugates targeted to primer binding site of HIV-1 genome
Abstract
We have shown that polyamide nucleic acids (PNAs) targeted to the PBS (PNA(PBS)) and A-loop (PNA(A-loop)) sequences, when transfected into cells, inhibit HIV-1 replication by blocking the initiation of reverse transcription via destabilizing tRNA(3)(Lys) primer from the viral genome. Here we demonstrate that both PNA(PBS) and PNA(A-loop) conjugated with the membrane-transducing peptide (MTD) vectors penetratin and Tat are rapidly taken up by cells and inhibit HIV-1 replication. Moreover, MTD peptide conjugates of PNA(PBS) and PNA(A-loop) displayed potent virucidal activity against HIV-1. Brief exposure of HIV-1 virions to these conjugates rendered them noninfectious. The IC(50) values for virucidal activity were in the range of approximately 50 nM; IC(50) values for inhibition of HIV-1 replication/infection were 0.5 microM-0.7 microM. The virucidal property of these conjugates suggests that a cocktail of anti-HIV-1 PNA-MTD peptide conjugates targeting critical regions of the HIV-1 genome could serve as a prophylactic agent for inactivating HIV-1 virions after exposure to HIV-1.
Figures







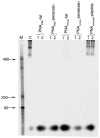
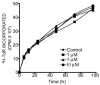
Similar articles
-
Anti-HIV-1 activity of anti-TAR polyamide nucleic acid conjugated with various membrane transducing peptides.Nucleic Acids Res. 2005 Aug 2;33(13):4345-56. doi: 10.1093/nar/gki743. Print 2005. Nucleic Acids Res. 2005. PMID: 16077030 Free PMC article.
-
Pharmacokinetic analysis of polyamide nucleic-acid-cell penetrating peptide conjugates targeted against HIV-1 transactivation response element.Oligonucleotides. 2008 Sep;18(3):277-86. doi: 10.1089/oli.2008.0140. Oligonucleotides. 2008. PMID: 18729823 Free PMC article.
-
Inhibition of HIV-1 replication by anti-trans-activation responsive polyamide nucleotide analog.Antiviral Res. 2002 Oct;56(1):13-27. doi: 10.1016/s0166-3542(02)00024-4. Antiviral Res. 2002. PMID: 12323396
-
HIV-1 inactivation by nucleic acid aptamers.Front Biosci. 2006 Jan 1;11:89-112. doi: 10.2741/1782. Front Biosci. 2006. PMID: 16146716 Review.
-
Inhibitors of HIV-1 gene expression and transcription.Curr Top Med Chem. 2004;4(9):871-82. doi: 10.2174/1568026043388466. Curr Top Med Chem. 2004. PMID: 15134546 Review.
Cited by
-
Cell-Penetrating Peptides for Antiviral Drug Development.Pharmaceuticals (Basel). 2010 Mar 2;3(3):448-470. doi: 10.3390/ph3030448. Pharmaceuticals (Basel). 2010. PMID: 27713263 Free PMC article. Review.
-
Development and application of ribonucleic acid therapy strategies against COVID-19.Int J Biol Sci. 2022 Aug 1;18(13):5070-5085. doi: 10.7150/ijbs.72706. eCollection 2022. Int J Biol Sci. 2022. PMID: 35982905 Free PMC article. Review.
-
Prospects for antisense peptide nucleic acid (PNA) therapies for HIV.Expert Opin Biol Ther. 2009 Aug;9(8):975-89. doi: 10.1517/14712590903052877. Expert Opin Biol Ther. 2009. PMID: 19534584 Free PMC article. Review.
-
An efficient biodelivery system for antisense polyamide nucleic acid (PNA).Oligonucleotides. 2008 Sep;18(3):245-56. doi: 10.1089/oli.2008.0126. Oligonucleotides. 2008. PMID: 18707540 Free PMC article.
-
Novel aptamer inhibitors of human immunodeficiency virus reverse transcriptase.Oligonucleotides. 2008 Jun;18(2):133-44. doi: 10.1089/oli.2008.0103. Oligonucleotides. 2008. PMID: 18637731 Free PMC article.
References
-
- Bourinbaiar AS, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9, and gossypol. Contraception. 1994;49:131–137. - PubMed
-
- Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. - PubMed
-
- Chaubey B, Tripathi S, Ganguly S, Harris D, Casale RA, Pandey VN. A PNA-transportan conjugate targeted to the TAR region of the HIV-1 genome exhibits both antiviral and virucidal properties. Virology. 2005;331:418–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

