Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience
- PMID: 17315156
- PMCID: PMC2952542
- DOI: 10.1002/cncr.22508
Survival advantage with decitabine versus intensive chemotherapy in patients with higher risk myelodysplastic syndrome: comparison with historical experience
Abstract
Background: Decitabine, a hypomethylating agent, is active and has been approved for the treatment of myelodysplastic syndrome (MDS) and chronic myelomonocytic leukemia. Intensive chemotherapy is an accepted form of therapy for patients with higher risk MDS. The comparative efficacy of these 2 forms of treatment in MDS is unknown. The objective of the current study was to compare the efficacy and toxicity profiles of decitabine and intensive chemotherapy in MDS.
Methods: The authors compared lower intensity decitabine therapy (n = 115 patients) with intensive chemotherapy (as it is used in acute myeloid leukemia [AML]) in patients with higher risk MDS. Two comparisons were made with a cohort of 376 historic patients (from 1995 to 2005): The first comparison included a subcohort of 115 patients (Group A) who matched the 115 decitabine study patients according to age, International Prognostic Scoring System, and cytogenetics; and the second comparison included the whole cohort of 376 patients without matching (Group B). A multivariate analysis was performed for outcome.
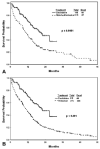
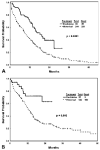
Results: The complete remission (CR) rate according to AML criteria was 43% with decitabine, 46% with intensive chemotherapy in Group A, and 52% with intensive chemotherapy in Group B. Compared with Group A, mortality at 6 weeks was 3% with decitabine versus 13% with intensive chemotherapy (P = .006) and, at 3 months, 7% with decitabine versus 23% with intensive chemotherapy (P = .001). Survival was better with decitabine versus intensive chemotherapy in Group A (median survival: 22 months vs 12 months; P < .001). A multivariate analysis of survival in all 491 patients who received decitabine or intensive chemotherapy (Group B) selected decitabine as an independent, favorable prognostic factor for survival (P = .006; hazard ratio, 0.74) after accounting for the independent prognostic effect of pretreatment factors.
Conclusions: In this analysis, decitabine was associated with a survival advantage compared with intensive chemotherapy in patients with higher risk MDS. Future studies should evaluate prospectively the results of decitabine versus intensive chemotherapy in this setting.
Figures
Similar articles
-
Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome.Cancer. 2007 Jan 15;109(2):265-73. doi: 10.1002/cncr.22376. Cancer. 2007. PMID: 17133405 Clinical Trial.
-
Comparison of Azacitidine and Decitabine in Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Network Meta-analysis.Clin Lymphoma Myeloma Leuk. 2021 Jun;21(6):e530-e544. doi: 10.1016/j.clml.2021.01.024. Epub 2021 Feb 24. Clin Lymphoma Myeloma Leuk. 2021. PMID: 33716056
-
Results of phase 2 randomized study of low-dose decitabine with or without valproic acid in patients with myelodysplastic syndrome and acute myelogenous leukemia.Cancer. 2015 Feb 15;121(4):556-61. doi: 10.1002/cncr.29085. Epub 2014 Oct 21. Cancer. 2015. PMID: 25336333 Free PMC article. Clinical Trial.
-
Comparison between decitabine and azacitidine for the treatment of myelodysplastic syndrome: a meta-analysis with 1,392 participants.Clin Lymphoma Myeloma Leuk. 2015 Jan;15(1):22-8. doi: 10.1016/j.clml.2014.04.010. Epub 2014 Jun 12. Clin Lymphoma Myeloma Leuk. 2015. PMID: 25042977 Review.
-
Epigenetics in clinical practice: the examples of azacitidine and decitabine in myelodysplasia and acute myeloid leukemia.Leukemia. 2013 Sep;27(9):1803-12. doi: 10.1038/leu.2013.173. Epub 2013 Jun 12. Leukemia. 2013. PMID: 23757301 Review.
Cited by
-
Platelet response during the second cycle of decitabine treatment predicts response and survival for myelodysplastic syndrome patients.Oncotarget. 2015 Jun 30;6(18):16653-62. doi: 10.18632/oncotarget.3914. Oncotarget. 2015. PMID: 25938546 Free PMC article.
-
Epigenetic-based therapies in cancer: progress to date.Drugs. 2011 Dec 24;71(18):2391-403. doi: 10.2165/11596690-000000000-00000. Drugs. 2011. PMID: 22141383 Review.
-
The revolution of myelodysplastic syndromes.Ther Adv Hematol. 2011 Feb;2(1):33-43. doi: 10.1177/2040620710395652. Ther Adv Hematol. 2011. PMID: 23556074 Free PMC article.
-
A phase 1-2 study of a farnesyltransferase inhibitor, tipifarnib, combined with idarubicin and cytarabine for patients with newly diagnosed acute myeloid leukemia and high-risk myelodysplastic syndrome.Cancer. 2011 Mar 15;117(6):1236-44. doi: 10.1002/cncr.25575. Epub 2010 Oct 19. Cancer. 2011. PMID: 20960519 Free PMC article. Clinical Trial.
-
5-azacitidine prolongs overall survival in patients with myelodysplastic syndrome--a systematic review and meta-analysis.Haematologica. 2010 Feb;95(2):303-10. doi: 10.3324/haematol.2009.010611. Epub 2009 Sep 22. Haematologica. 2010. PMID: 19773261 Free PMC article.
References
-
- Faderl S, Kantarjian H. Novel therapies for myelodysplastic syndromes. Cancer. 2004;101:226–241. - PubMed
-
- Faderl S, Kantarjian H. Myelodysplastic syndromes. In: DeVita V, Hellman S, Rosenberg S, editors. Cancer: Principles and Practice of Oncology. 7th. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 2144–2154.
-
- Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. - PubMed
-
- Harris NJ, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting, Airlie House, Virginia, November 1977. J Clin Oncol. 1999;17:3835–3849. - PubMed
-
- Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous