C-terminal Src kinase controls development and maintenance of mouse squamous epithelia
- PMID: 17304209
- PMCID: PMC1817640
- DOI: 10.1038/sj.emboj.7601595
C-terminal Src kinase controls development and maintenance of mouse squamous epithelia
Abstract
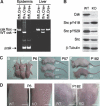
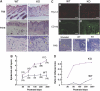
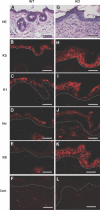
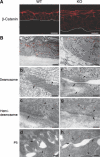
Carboxy-terminal Src kinase (Csk) is a negative regulator of Src family kinases, which play pivotal roles in controlling cell adhesion, migration, and cancer progression. To elucidate the in vivo role of Csk in epithelial tissues, we conditionally inactivated Csk in squamous epithelia using the keratin-5 promoter/Cre-loxP system in mice. The mutant mice developed apparent defects in the skin, esophagus, and forestomach, with concomitant hyperplasia and chronic inflammation. Histology of the mutant epidermis revealed impaired cell-cell adhesion in basal cell layers. Analysis of primary keratinocytes showed that the defective cell-cell adhesion was caused by cytoskeletal remodeling via activation of the Rac1 pathway. Mutant keratinocytes also showed elevated expression of mesenchymal proteins, matrix metalloproteinases (MMPs), and the proinflammatory cytokine TNF-alpha. Inhibition of the expression of TNF-alpha and MMP9 by the anti-inflammatory reagent FK506 could cure the epidermal hyperplasia, suggesting a causal link between inflammation and epidermal hyperplasia. These observations demonstrate that the Src/Csk circuit plays crucial roles in development and maintenance of epithelia by controlling cytoskeletal organization as well as phenotypic conversion linked to inflammatory events.
Figures
Similar articles
-
Ablation of Csk in neural crest lineages causes corneal anomaly by deregulating collagen fibril organization and cell motility.Dev Biol. 2008 Mar 15;315(2):474-88. doi: 10.1016/j.ydbio.2008.01.004. Epub 2008 Jan 16. Dev Biol. 2008. PMID: 18262517
-
Suprabasal Dsg2 expression in transgenic mouse skin confers a hyperproliferative and apoptosis-resistant phenotype to keratinocytes.J Cell Sci. 2007 Mar 1;120(Pt 5):758-71. doi: 10.1242/jcs.03392. Epub 2007 Feb 6. J Cell Sci. 2007. PMID: 17284515
-
Essential roles of Lyn in fibronectin-mediated filamentous actin assembly and cell motility in mast cells.J Immunol. 1998 Oct 1;161(7):3694-701. J Immunol. 1998. PMID: 9759894
-
C-terminal Src kinase (CSK) and CSK-homologous kinase (CHK)--endogenous negative regulators of Src-family protein kinases.Growth Factors. 2005 Sep;23(3):233-44. doi: 10.1080/08977190500178877. Growth Factors. 2005. PMID: 16243715 Review.
-
Knockouts of Src-family kinases: stiff bones, wimpy T cells, and bad memories.Genes Dev. 1996 Aug 1;10(15):1845-57. doi: 10.1101/gad.10.15.1845. Genes Dev. 1996. PMID: 8756343 Review. No abstract available.
Cited by
-
Transforming potential of Src family kinases is limited by the cholesterol-enriched membrane microdomain.Mol Cell Biol. 2009 Dec;29(24):6462-72. doi: 10.1128/MCB.00941-09. Epub 2009 Oct 12. Mol Cell Biol. 2009. PMID: 19822664 Free PMC article.
-
A vertebrate-specific Chp-PAK-PIX pathway maintains E-cadherin at adherens junctions during zebrafish epiboly.PLoS One. 2010 Apr 12;5(4):e10125. doi: 10.1371/journal.pone.0010125. PLoS One. 2010. PMID: 20405038 Free PMC article.
-
An adaptor role for cytoplasmic Sam68 in modulating Src activity during cell polarization.Mol Cell Biol. 2009 Apr;29(7):1933-43. doi: 10.1128/MCB.01707-08. Epub 2009 Jan 12. Mol Cell Biol. 2009. PMID: 19139276 Free PMC article.
-
Inducible deletion of epidermal Dicer and Drosha reveals multiple functions for miRNAs in postnatal skin.Development. 2012 Apr;139(8):1405-16. doi: 10.1242/dev.070920. Development. 2012. PMID: 22434867 Free PMC article.
-
Src is activated by the nuclear receptor peroxisome proliferator-activated receptor β/δ in ultraviolet radiation-induced skin cancer.EMBO Mol Med. 2014 Jan;6(1):80-98. doi: 10.1002/emmm.201302666. EMBO Mol Med. 2014. PMID: 24203162 Free PMC article.
References
-
- Aki D, Mashima R, Saeki K, Minoda Y, Yamauchi M, Yoshimura A (2005) Modulation of TLR signalling by the C-terminal Src kinase (Csk) in macrophages. Genes Cells 10: 357–368 - PubMed
-
- Avizienyte E, Frame MC (2005) Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol 17: 542–547 - PubMed
-
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel MM, Birchmeier W (1993) Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J Cell Biol 120: 757–766 - PMC - PubMed
-
- Braga VM, Yap AS (2005) The challenges of abundance: epithelial junctions and small GTPase. Curr Opin Cell Biol 17: 466–474 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

