Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking
- PMID: 17301289
- PMCID: PMC1838986
- DOI: 10.1091/mbc.e06-07-0593
Cytokine stimulation promotes glucose uptake via phosphatidylinositol-3 kinase/Akt regulation of Glut1 activity and trafficking
Abstract
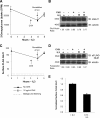
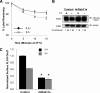
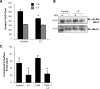
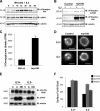
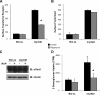
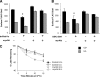
Cells require growth factors to support glucose metabolism for survival and growth. It is unclear, however, how noninsulin growth factors may regulate glucose uptake and glucose transporters. We show that the hematopoietic growth factor interleukin (IL)3, maintained the glucose transporter Glut1 on the cell surface and promoted Rab11a-dependent recycling of intracellular Glut1. IL3 required phosphatidylinositol-3 kinase activity to regulate Glut1 trafficking, and activated Akt was sufficient to maintain glucose uptake and surface Glut1 in the absence of IL3. To determine how Akt may regulate Glut1, we analyzed the role of Akt activation of mammalian target of rapamycin (mTOR)/regulatory associated protein of mTOR (RAPTOR) and inhibition of glycogen synthase kinase (GSK)3. Although Akt did not require mTOR/RAPTOR to maintain surface Glut1 levels, inhibition of mTOR/RAPTOR by rapamycin greatly diminished glucose uptake, suggesting Akt-stimulated mTOR/RAPTOR may promote Glut1 transporter activity. In contrast, inhibition of GSK3 did not affect Glut1 internalization but nevertheless maintained surface Glut1 levels in IL3-deprived cells, possibly via enhanced recycling of internalized Glut1. In addition, Akt attenuated Glut1 internalization through a GSK3-independent mechanism. These data demonstrate that intracellular trafficking of Glut1 is a regulated component of growth factor-stimulated glucose uptake and that Akt can promote Glut1 activity and recycling as well as prevent Glut1 internalization.
Figures

Similar articles
-
A GSK-3/TSC2/mTOR pathway regulates glucose uptake and GLUT1 glucose transporter expression.Am J Physiol Cell Physiol. 2008 Sep;295(3):C836-43. doi: 10.1152/ajpcell.00554.2007. Epub 2008 Jul 23. Am J Physiol Cell Physiol. 2008. PMID: 18650261 Free PMC article.
-
Enhanced expression of glucose transporter-1 in vascular smooth muscle cells via the Akt/tuberous sclerosis complex subunit 2 (TSC2)/mammalian target of rapamycin (mTOR)/ribosomal S6 protein kinase (S6K) pathway in experimental renal failure.J Vasc Surg. 2013 Feb;57(2):475-85. doi: 10.1016/j.jvs.2012.07.037. Epub 2012 Dec 21. J Vasc Surg. 2013. PMID: 23265586
-
Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes.Endocrinology. 2005 Mar;146(3):1328-37. doi: 10.1210/en.2004-0777. Epub 2004 Dec 2. Endocrinology. 2005. PMID: 15576463
-
Roles of glucose transporter-1 and the phosphatidylinositol 3‑kinase/protein kinase B pathway in cancer radioresistance (review).Mol Med Rep. 2015 Mar;11(3):1573-81. doi: 10.3892/mmr.2014.2888. Epub 2014 Nov 6. Mol Med Rep. 2015. PMID: 25376370 Review.
-
Glut 1 in Cancer Cells and the Inhibitory Action of Resveratrol as A Potential Therapeutic Strategy.Int J Mol Sci. 2019 Jul 9;20(13):3374. doi: 10.3390/ijms20133374. Int J Mol Sci. 2019. PMID: 31324056 Free PMC article. Review.
Cited by
-
Genetic heterogeneity of diffuse large B-cell lymphoma.Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):1398-403. doi: 10.1073/pnas.1205299110. Epub 2013 Jan 4. Proc Natl Acad Sci U S A. 2013. PMID: 23292937 Free PMC article.
-
Metabolic regulation of T lymphocytes.Annu Rev Immunol. 2013;31:259-83. doi: 10.1146/annurev-immunol-032712-095956. Epub 2013 Jan 3. Annu Rev Immunol. 2013. PMID: 23298210 Free PMC article. Review.
-
Signal transduction in cancer.Cold Spring Harb Perspect Med. 2015 Apr 1;5(4):a006098. doi: 10.1101/cshperspect.a006098. Cold Spring Harb Perspect Med. 2015. PMID: 25833940 Free PMC article. Review.
-
Changing the energy of an immune response.Am J Clin Exp Immunol. 2013 Feb 27;2(1):30-54. Print 2013. Am J Clin Exp Immunol. 2013. PMID: 23885324 Free PMC article.
-
Membrane transporters in cell physiology, cancer metabolism and drug response.Dis Model Mech. 2023 Nov 1;16(11):dmm050404. doi: 10.1242/dmm.050404. Epub 2023 Dec 1. Dis Model Mech. 2023. PMID: 38037877 Free PMC article. Review.
References
-
- Asano T., et al. The role of N-glycosylation of GLUT1 for glucose transport activity. J. Biol. Chem. 1991;266:24632–24636. - PubMed
-
- Barnes K., McIntosh E., Whetton A. D., Daley G. Q., Bentley J., Baldwin S. A. Chronic myeloid leukaemia: an investigation into the role of Bcr-Abl-induced abnormalities in glucose transport regulation. Oncogene. 2005;24:3257–3267. - PubMed
-
- Barthel A., Okino S. T., Liao J., Nakatani K., Li J., Whitlock J. P., Jr, Roth R. A. Regulation of GLUT1 gene transcription by the serine/threonine kinase Akt1. J. Biol. Chem. 1999;274:20281–20286. - PubMed
-
- Bentley J., Itchayanan D., Barnes K., McIntosh E., Tang X., Downes C. P., Holman G. D., Whetton A. D., Owen-Lynch P. J., Baldwin S. A. Interleukin-3-mediated cell survival signals include phosphatidylinositol 3-kinase-dependent translocation of the glucose transporter GLUT1 to the cell surface. J. Biol. Chem. 2003;278:39337–39348. - PubMed
-
- Cham C. M., Gajewski T. F. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 2005;174:4670–4677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

