The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis
- PMID: 17299048
- PMCID: PMC1815247
- DOI: 10.1073/pnas.0610296104
The EGF repeat and discoidin domain protein, SED1/MFG-E8, is required for mammary gland branching morphogenesis
Abstract
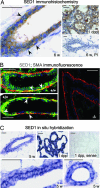
SED1, also known as MFG-E8, is a secreted protein composed of two EGF repeats (the second of which contains an RGD motif) and two discoidin/Factor V/VIII C domains. SED1 is expressed by a wide range of cell types, where it participates in diverse cellular interactions, such as sperm binding to the egg coat and macrophage recognition of apoptotic lymphocytes. Although SED1 was originally identified as a milk protein, its function in the mammary gland remains unclear; suggested functions include inhibition of viral infection and clearance of apoptotic cells during mammary gland involution. We report here that SED1 has an unexpected obligatory role during mammary gland development. Unlike that seen in WT glands, SED1-null glands show severely reduced branching from epithelial ducts and from terminal end buds, which are thin and poorly developed. SED1 is expressed by both luminal and myoepithelial cells in the developing epithelial duct, and binds to alpha(v) integrin receptors on myoepithelial cells leading to MAPK activation and cell proliferation. The absence of SED1 leads to greatly reduced levels of activated MAPK and a concomitant reduction in cell proliferation and branching throughout the epithelial tree. These results suggest that SED1 contributes, at least partly, to the intercellular signaling between luminal and myoepithelial cells that is required for branching morphogenesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions.J Cell Biochem. 2009 Apr 15;106(6):957-66. doi: 10.1002/jcb.22076. J Cell Biochem. 2009. PMID: 19204935 Free PMC article. Review.
-
Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding.Cell. 2003 Aug 22;114(4):405-17. doi: 10.1016/s0092-8674(03)00643-3. Cell. 2003. PMID: 12941270
-
Adhesion activity of fetal gonadal cells to EGF and discoidin domains of milk fat globule-EGF factor 8 (MFG-E8), a secreted integrin-binding protein which is transiently expressed in mouse early gonadogenesis.Anat Embryol (Berl). 2005 Jul;209(6):485-94. doi: 10.1007/s00429-005-0463-0. Epub 2005 May 13. Anat Embryol (Berl). 2005. PMID: 15891907
-
Weaning-induced expression of a milk-fat globule protein, MFG-E8, in mouse mammary glands, as demonstrated by the analyses of its mRNA, protein and phosphatidylserine-binding activity.Biochem J. 2006 Apr 1;395(1):21-30. doi: 10.1042/BJ20051459. Biochem J. 2006. PMID: 16401186 Free PMC article.
-
Novel gamete receptors that facilitate sperm adhesion to the egg coat.Soc Reprod Fertil Suppl. 2007;63:367-83. Soc Reprod Fertil Suppl. 2007. PMID: 17566285 Review.
Cited by
-
Impaired cell death and mammary gland involution in the absence of Dock1 and Rac1 signaling.Cell Death Dis. 2014 Aug 14;5(8):e1375. doi: 10.1038/cddis.2014.338. Cell Death Dis. 2014. PMID: 25118935 Free PMC article.
-
Fetal hematopoietic stem cells express MFG-E8 during mouse embryogenesis.Exp Mol Med. 2015 Jul 24;47(7):e174. doi: 10.1038/emm.2015.42. Exp Mol Med. 2015. PMID: 26206421 Free PMC article.
-
The integrin alpha(v)beta(3-5) ligand MFG-E8 is a p63/p73 target gene in triple-negative breast cancers but exhibits suppressive functions in ER(+) and erbB2(+) breast cancers.Cancer Res. 2011 Feb 1;71(3):937-45. doi: 10.1158/0008-5472.CAN-10-1471. Epub 2010 Dec 2. Cancer Res. 2011. PMID: 21127199 Free PMC article.
-
Loss of SED1/MFG-E8 results in altered luminal physiology in the epididymis.Mol Reprod Dev. 2010 Jun;77(6):550-63. doi: 10.1002/mrd.21189. Mol Reprod Dev. 2010. PMID: 20422713 Free PMC article.
-
Human mammospheres secrete hormone-regulated active extracellular vesicles.PLoS One. 2014 Jan 3;9(1):e83955. doi: 10.1371/journal.pone.0083955. eCollection 2014. PLoS One. 2014. PMID: 24404144 Free PMC article.
References
-
- Larocca D, Peterson JA, Urrea R, Kuniyoshi J, Bistrain AM, Ceriani RL. Cancer Res. 1991;51:4994–4998. - PubMed
-
- Couto JR, Taylor MR, Godwin SG, Ceriani RL, Peterson JA. DNA Cell Biol. 1996;15:281–286. - PubMed
-
- Andersen MH, Berglund L, Rasmussen JT, Petersen TE. Biochemistry. 1997;36:5441–5446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

