Differential expression of class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration
- PMID: 17278142
- PMCID: PMC3917508
- DOI: 10.1002/cne.21283
Differential expression of class 3 and 4 semaphorins and netrin in the lamprey spinal cord during regeneration
Abstract
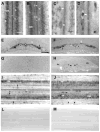
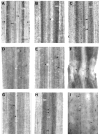
To explore the role of axon guidance molecules during regeneration in the lamprey spinal cord, we examined the expression of mRNAs for semaphorin 3 (Sema3), semaphorin 4 (Sema4), and netrin during regeneration by in situ hybridization. Control lampreys contained netrin-expressing neurons along the length of the spinal cord. After spinal transection, netrin expression was downregulated in neurons close (500 mum to 10 mm) to the transection at 2 and 4 weeks. A high level of Sema4 expression was found in the neurons of the gray matter and occasionally in the dorsal and the edge cells. Fourteen days after spinal cord transection Sema4 mRNA expression was absent from dorsal and edge cells but was still present in neurons of the gray matter. At 30 days the expression had declined to some extent in neurons and was absent in dorsal and edge cells. In control animals, Sema3 was expressed in neurons of the gray matter and in dorsal and edge cells. Two weeks after transection, Sema3 expression was upregulated near the lesion, but absent in dorsal cells. By 4 weeks a few neurons expressed Sema3 at 20 mm caudal to the transection but no expression was detected 1 mm from the transection. Isolectin I-B(4) labeling for microglia/macrophages showed that the number of Sema3-expressing microglia/macrophages increased dramatically at the injury site over time. The downregulation of netrin and upregulation of Sema3 near the transection suggests a possible role of netrin and semaphorins in restricting axonal regeneration in the injured spinal cord.
(c) 2007 Wiley-Liss, Inc.
Figures



Similar articles
-
Expression of neurotrophin and its tropomyosin-related kinase receptors (Trks) during axonal regeneration following spinal cord injury in larval lamprey.Neuroscience. 2011 Jun 2;183:265-77. doi: 10.1016/j.neuroscience.2011.03.024. Epub 2011 Mar 21. Neuroscience. 2011. PMID: 21421025 Free PMC article.
-
Expression of the netrin receptor UNC-5 in lamprey brain: modulation by spinal cord transection.Neurorehabil Neural Repair. 2000;14(1):49-58. doi: 10.1177/154596830001400106. Neurorehabil Neural Repair. 2000. PMID: 11228949
-
Expression of the repulsive guidance molecule RGM and its receptor neogenin after spinal cord injury in sea lamprey.Exp Neurol. 2009 Jun;217(2):242-51. doi: 10.1016/j.expneurol.2009.02.011. Epub 2009 Mar 5. Exp Neurol. 2009. PMID: 19268666 Free PMC article.
-
Role of Netrin-1 Signaling in Nerve Regeneration.Int J Mol Sci. 2017 Feb 24;18(3):491. doi: 10.3390/ijms18030491. Int J Mol Sci. 2017. PMID: 28245592 Free PMC article. Review.
-
Axonal guidance molecules and the failure of axonal regeneration in the adult mammalian spinal cord.Restor Neurol Neurosci. 2008;26(2-3):117-30. Restor Neurol Neurosci. 2008. PMID: 18820406 Review.
Cited by
-
Spontaneous regeneration of cholecystokinergic reticulospinal axons after a complete spinal cord injury in sea lampreys.Comput Struct Biotechnol J. 2023 Dec 19;23:347-357. doi: 10.1016/j.csbj.2023.12.014. eCollection 2024 Dec. Comput Struct Biotechnol J. 2023. PMID: 38205155 Free PMC article.
-
Brief Electrical Stimulation Triggers an Effective Regeneration of Leech CNS.eNeuro. 2020 Jun 25;7(3):ENEURO.0030-19.2020. doi: 10.1523/ENEURO.0030-19.2020. Print 2020 May/Jun. eNeuro. 2020. PMID: 32471846 Free PMC article.
-
The Composition and Cellular Sources of CSPGs in the Glial Scar After Spinal Cord Injury in the Lamprey.Front Mol Neurosci. 2022 Jun 27;15:918871. doi: 10.3389/fnmol.2022.918871. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35832392 Free PMC article.
-
Expression of neurotrophin and its tropomyosin-related kinase receptors (Trks) during axonal regeneration following spinal cord injury in larval lamprey.Neuroscience. 2011 Jun 2;183:265-77. doi: 10.1016/j.neuroscience.2011.03.024. Epub 2011 Mar 21. Neuroscience. 2011. PMID: 21421025 Free PMC article.
-
Lampreys and spinal cord regeneration: "a very special claim on the interest of zoologists," 1830s-present.Front Cell Dev Biol. 2023 May 9;11:1113961. doi: 10.3389/fcell.2023.1113961. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37228651 Free PMC article.
References
-
- Armstrong J, Zhang L, McClellan AD. Axonal regeneration of descending and ascending spinal projection neurons in spinal cord-transected larval lamprey. Exp Neurol. 2003;180:156–166. - PubMed
-
- Bagnard D, Lohrum M, Uziel D, Puschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. - PubMed
-
- Batchelor PE, Porritt MJ, Martinello P, Parish CL, Liberatore GT, Donnan GA, Howells DW. Macrophages and microglia produce local trophic gradients that stimulate axonal sprouting toward but not beyond the wound edge. Mol Cell Neurosci. 2002;21:436–453. - PubMed
-
- Boya J, Carbonell AL, Calvo JL, Borregon A. Microglial cells in the central nervous system of the rabbit and rat: cytochemical identification using two different lectins. Acta Anat (Basel) 1991;140:250–253. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS038537/NS/NINDS NIH HHS/United States
- R24 HD050838-01/HD/NICHD NIH HHS/United States
- R01 NS038537-09/NS/NINDS NIH HHS/United States
- R01 NS038537-01A1/NS/NINDS NIH HHS/United States
- R01 NS038537-03/NS/NINDS NIH HHS/United States
- R01 NS38537/NS/NINDS NIH HHS/United States
- R24 HD050838-02/HD/NICHD NIH HHS/United States
- R01 NS038537-02/NS/NINDS NIH HHS/United States
- R01 NS038537-06/NS/NINDS NIH HHS/United States
- R01 NS038537-05/NS/NINDS NIH HHS/United States
- R24 HD050838/HD/NICHD NIH HHS/United States
- R01 NS038537-10/NS/NINDS NIH HHS/United States
- R24 HD050838-03/HD/NICHD NIH HHS/United States
- R01 NS038537-07/NS/NINDS NIH HHS/United States
- R24 HD050838-04/HD/NICHD NIH HHS/United States
- R24 HD050838-05/HD/NICHD NIH HHS/United States
- R01 NS038537-04/NS/NINDS NIH HHS/United States
- R01 NS038537-08/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

