Lack of NF-kappaB1 (p105/p50) attenuates unloading-induced downregulation of PPARalpha and PPARalpha-regulated gene expression in rodent heart
- PMID: 17276423
- PMCID: PMC2034318
- DOI: 10.1016/j.cardiores.2006.12.021
Lack of NF-kappaB1 (p105/p50) attenuates unloading-induced downregulation of PPARalpha and PPARalpha-regulated gene expression in rodent heart
Abstract
Objective: Unloading of the rodent heart activates the fetal gene program, decreases peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARalpha-regulated gene expression (MCAD), and induces cardiomyocyte atrophy. NF-kappaB regulates the fetal gene program and PPARalpha-regulated gene expression during cardiac hypertrophy and induces atrophy in skeletal muscle. Our objective was to test the hypothesis that NF-kappaB is the regulator for activation of the fetal gene program, for downregulation of PPARalpha and PPARalpha-regulated gene expression, and for cardiomyocyte atrophy in the heart subjected to mechanical unloading.
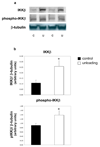
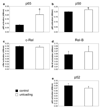
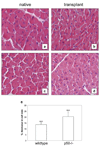
Methods: Activation of the inhibitory kappa B kinase beta (IKKbeta)/NF-kappaB pathways were measured in the heterotopically transplanted rat heart using Western blotting of total and phospho-IKKbeta and using transcription factor ELISA's for the five members of the NF-kappaB family (p65 (Rel A), p105/p50, c-Rel, RelB, and p100/p52). In loss of function experiments, we transplanted hearts of p105/p50 knockout mice into wildtype mice and compared changes in gene expression and cardiomyocyte size with wildtype hearts transplanted into wildtype mice.
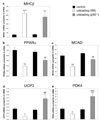
Results: Total and phospho-IKKbeta levels significantly increased in the transplanted heart seven days after surgery. The activation of IKKbeta was paralleled by increased DNA binding activity of p65 and p105/p50. Mechanical unloading induced myosin heavy chain beta expression and decreased cardiomyocyte size in hearts of both wildtype and p105/p050 knockout animals. In contrast, the downregulation of PPARalpha and MCAD was significantly attenuated or prevented in the hearts of p105/p50 knockout mice.
Conclusions: The IKKbeta/p65/p50 pathway is activated in the unloaded rodent heart and a likely regulator for the downregulation of PPARalpha and PPARalpha-regulated gene expression.
Figures
Similar articles
-
Neuroadaptations in human chronic alcoholics: dysregulation of the NF-kappaB system.PLoS One. 2007 Sep 26;2(9):e930. doi: 10.1371/journal.pone.0000930. PLoS One. 2007. PMID: 17895971 Free PMC article.
-
Tumour necrosis factor alpha down-regulates the expression of peroxisome proliferator activated receptor alpha (PPARα) in human hepatocarcinoma HepG2 cells by activation of NF-κB pathway.Cytokine. 2013 Jan;61(1):266-74. doi: 10.1016/j.cyto.2012.10.007. Epub 2012 Nov 8. Cytokine. 2013. PMID: 23141142
-
A roadmap of constitutive NF-κB activity in Hodgkin lymphoma: Dominant roles of p50 and p52 revealed by genome-wide analyses.Genome Med. 2016 Mar 17;8(1):28. doi: 10.1186/s13073-016-0280-5. Genome Med. 2016. PMID: 26988706 Free PMC article.
-
Nuclear factor-kappaB1: regulation and function.Int J Biochem Cell Biol. 2008;40(8):1425-30. doi: 10.1016/j.biocel.2007.05.004. Epub 2007 May 17. Int J Biochem Cell Biol. 2008. PMID: 17693123 Review.
-
Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology.Biochem J. 2004 Sep 1;382(Pt 2):393-409. doi: 10.1042/BJ20040544. Biochem J. 2004. PMID: 15214841 Free PMC article. Review.
Cited by
-
Cardiac Complications: The Understudied Aspect of Cancer Cachexia.Cardiovasc Toxicol. 2022 Mar;22(3):254-267. doi: 10.1007/s12012-022-09727-9. Epub 2022 Feb 16. Cardiovasc Toxicol. 2022. PMID: 35171467 Review.
-
The impairment of ILK related angiogenesis involved in cardiac maladaptation after infarction.PLoS One. 2011;6(9):e24115. doi: 10.1371/journal.pone.0024115. Epub 2011 Sep 16. PLoS One. 2011. PMID: 21949693 Free PMC article.
-
SOD1 overexpression in paraventricular nucleus improves post-infarct myocardial remodeling and ventricular function.Pflugers Arch. 2012 Feb;463(2):297-307. doi: 10.1007/s00424-011-1036-0. Epub 2011 Oct 18. Pflugers Arch. 2012. PMID: 22006090
-
PPARα is down-regulated following liver transplantation in mice.J Hepatol. 2012 Mar;56(3):586-94. doi: 10.1016/j.jhep.2011.08.021. Epub 2011 Oct 25. J Hepatol. 2012. PMID: 22037025 Free PMC article.
-
Dynamic patterns of ventricular remodeling and apoptosis in hearts unloaded by heterotopic transplantation.J Heart Lung Transplant. 2014 Feb;33(2):203-10. doi: 10.1016/j.healun.2013.10.006. Epub 2013 Oct 4. J Heart Lung Transplant. 2014. PMID: 24315785 Free PMC article.
References
-
- Depre C, Shipley GL, Chen W, Han Q, Doenst T, Moore ML, et al. Unloaded heart in vivo replicates fetal gene expression of cardiac hypertrophy. Nat Med. 1998;4:1269–1275. - PubMed
-
- Sharma S, Ying J, Razeghi P, Stepkowski S, Taegtmeyer H. Atrophic remodeling of the transplanted rat heart. Cardiology. 2006;105:128–136. - PubMed
-
- Razeghi P, Sharma S, Ying J, Li YP, Stepkowski S, Reid MB, et al. Atrophic remodeling of the heart in vivo simultaneously activates pathways of protein synthesis and degradation. Circulation. 2003;108:2536–2541. - PubMed
-
- Thanos D, Maniatis T. Nf-κb: A lesson in family values. Cell. 1995;80:529–532. - PubMed
-
- Freund C, Schmidt-Ullrich R, Baurand A, Dunger S, Schneider W, Loser P, et al. Requirement of nuclear factor-κb in angiotensin ii- and isoproterenol-induced cardiac hypertrophy in vivo. Circulation. 2005;111:2319–2325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

