Novel regulation of MHC class II function in B cells
- PMID: 17255932
- PMCID: PMC1794403
- DOI: 10.1038/sj.emboj.7601556
Novel regulation of MHC class II function in B cells
Abstract
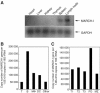
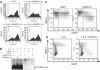
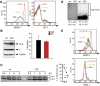
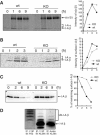
The presence of post-translational regulation of MHC class II (MHC II) under physiological conditions has been demonstrated recently in dendritic cells (DCs) that potently function as antigen-presenting cells (APCs). Here, we report that MARCH-I, an E3 ubiquitin ligase, plays a pivotal role in the post-translational regulation of MHC II in B cells. MARCH-I expression was particularly high in B cells, and the forced expression of MARCH-I induced the ubiquitination of MHC II. In B cells from MARCH-I-deficient mice (MARCH-I KO), the half-life of surface MHC II was prolonged and the ubiquitinated form of MHC II completely disappeared. In addition, MARCH-I-deficient B cells highly expressed exogenous antigen-loaded MHC II on their surface and showed high ability to present exogenous antigens. These results suggest that the function of MHC II in B cells is regulated through ubiquitination by MARCH-I.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Enabling Systemic Identification and Functionality Profiling for Cdc42 Homeostatic Modulators.bioRxiv [Preprint]. 2024 Jan 8:2024.01.05.574351. doi: 10.1101/2024.01.05.574351. bioRxiv. 2024. Update in: Commun Chem. 2024 Nov 19;7(1):271. doi: 10.1038/s42004-024-01352-7. PMID: 38260445 Free PMC article. Updated. Preprint.
-
Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I.Elife. 2020 May 20;9:e55845. doi: 10.7554/eLife.55845. Elife. 2020. PMID: 32432547 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
-
Molecular control of steady-state dendritic cell maturation and immune homeostasis.Annu Rev Immunol. 2013;31:743-91. doi: 10.1146/annurev-immunol-020711-074929. Epub 2013 Jan 17. Annu Rev Immunol. 2013. PMID: 23330953 Free PMC article. Review.
-
Francisella tularensis elicits IL-10 via a PGE₂-inducible factor, to drive macrophage MARCH1 expression and class II down-regulation.PLoS One. 2012;7(5):e37330. doi: 10.1371/journal.pone.0037330. Epub 2012 May 17. PLoS One. 2012. PMID: 22615981 Free PMC article.
-
MARCH1-mediated MHCII ubiquitination promotes dendritic cell selection of natural regulatory T cells.J Exp Med. 2013 Jun 3;210(6):1069-77. doi: 10.1084/jem.20122695. Epub 2013 May 27. J Exp Med. 2013. PMID: 23712430 Free PMC article.
-
Molecular mechanism and cellular function of MHCII ubiquitination.Immunol Rev. 2015 Jul;266(1):134-44. doi: 10.1111/imr.12303. Immunol Rev. 2015. PMID: 26085212 Free PMC article. Review.
-
Early activation of teleost B cells in response to rhabdovirus infection.J Virol. 2015 Feb;89(3):1768-80. doi: 10.1128/JVI.03080-14. Epub 2014 Nov 19. J Virol. 2015. PMID: 25410870 Free PMC article.
References
-
- Coscoy L, Ganem D (2003) PHD domains and E3 ubiquitin ligases: viruses make the connection. Trends Cell Biol 13: 7–12 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

