Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells
- PMID: 17239373
- PMCID: PMC2040298
- DOI: 10.1016/j.yexcr.2006.12.005
Erk1/2 MAPK and caldesmon differentially regulate podosome dynamics in A7r5 vascular smooth muscle cells
Abstract
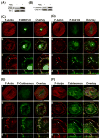
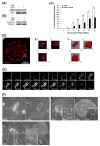
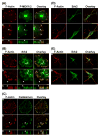
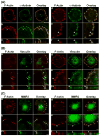
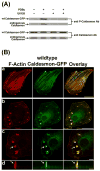
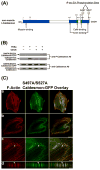
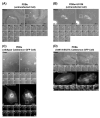
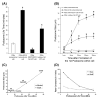
We tested the hypothesis that the MEK/Erk/caldesmon phosphorylation cascade regulates PKC-mediated podosome dynamics in A7r5 cells. We observed the phosphorylation of MEK, Erk and caldesmon, and their translocation to the podosomes upon phorbol dibutyrate (PDBu) stimulation, together with the nuclear translocation of phospho-MEK and phospho-Erk. After MEK inhibition by U0126, Erk translocated to the interconnected actin-rich columns but failed to translocate to the nucleus, suggesting that podosomes served as a site for Erk phosphorylation. The interconnected actin-rich columns in U0126-treated, PDBu-stimulated cells contained alpha-actinin, caldesmon, vinculin, and metalloproteinase-2. Caldesmon and vinculin became integrated with F-actin at the columns, in contrast to their typical location at the ring of podosomes. Live-imaging experiments suggested the growth of these columns from podosomes that were slow to disassemble. The observed modulation of podosome size and life time in A7r5 cells overexpressing wild-type and phosphorylation-deficient caldesmon-GFP mutants in comparison to untransfected cells suggests that caldesmon and caldesmon phosphorylation modulate podosome dynamics in A7r5 cells. These results suggest that Erk1/2 and caldesmon differentially modulate PKC-mediated formation and/or dynamics of podosomes in A7r5 vascular smooth muscle cells.
Figures
Similar articles
-
Caldesmon is an integral component of podosomes in smooth muscle cells.J Cell Sci. 2006 May 1;119(Pt 9):1691-702. doi: 10.1242/jcs.02881. Epub 2006 Apr 4. J Cell Sci. 2006. PMID: 16595550
-
Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in a7r5 smooth muscle cells.Exp Cell Res. 2002 Oct 15;280(1):64-74. doi: 10.1006/excr.2002.5592. Exp Cell Res. 2002. PMID: 12372340
-
Effects of tyrosine phosphorylation of cortactin on podosome formation in A7r5 vascular smooth muscle cells.Am J Physiol Cell Physiol. 2006 Feb;290(2):C463-71. doi: 10.1152/ajpcell.00350.2005. Epub 2005 Sep 14. Am J Physiol Cell Physiol. 2006. PMID: 16162656
-
Caldesmon phosphorylation in actin cytoskeletal remodeling.Eur J Cell Biol. 2006 Apr;85(3-4):305-9. doi: 10.1016/j.ejcb.2005.08.008. Epub 2005 Sep 12. Eur J Cell Biol. 2006. PMID: 16546574 Review.
-
p53 regulation of podosome formation and cellular invasion in vascular smooth muscle cells.Cell Adh Migr. 2011 Mar-Apr;5(2):144-9. doi: 10.4161/cam.5.2.14375. Epub 2011 Mar 1. Cell Adh Migr. 2011. PMID: 21164280 Free PMC article. Review.
Cited by
-
Leupaxin stimulates adhesion and migration of prostate cancer cells through modulation of the phosphorylation status of the actin-binding protein caldesmon.Oncotarget. 2015 May 30;6(15):13591-606. doi: 10.18632/oncotarget.3792. Oncotarget. 2015. PMID: 26079947 Free PMC article.
-
Integrins traffic rapidly via circular dorsal ruffles and macropinocytosis during stimulated cell migration.J Cell Biol. 2011 Apr 4;193(1):61-70. doi: 10.1083/jcb.201007003. J Cell Biol. 2011. PMID: 21464228 Free PMC article.
-
Caldesmon regulates the motility of vascular smooth muscle cells by modulating the actin cytoskeleton stability.J Biomed Sci. 2010 Feb 3;17(1):6. doi: 10.1186/1423-0127-17-6. J Biomed Sci. 2010. PMID: 20128924 Free PMC article.
-
Caldesmon as a therapeutic target for proliferative vascular diseases.Mini Rev Med Chem. 2008 Oct;8(12):1209-13. doi: 10.2174/138955708786140981. Mini Rev Med Chem. 2008. PMID: 18855735 Free PMC article. Review.
-
Invadosomes are coming: new insights into function and disease relevance.FEBS J. 2018 Jan;285(1):8-27. doi: 10.1111/febs.14123. Epub 2017 Jun 22. FEBS J. 2018. PMID: 28548369 Free PMC article. Review.
References
-
- Newby AC. Dual role of matrix metalloproteinases (Matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. - PubMed
-
- Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. - PubMed
-
- Burgstaller G, Gimona M. Podosome-mediated matrix resorption and cell motility in vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;288:3001–3005. - PubMed
-
- Hai CM, Hahne P, Harrington EO, Gimona M. Conventional protein kinase C mediates phorbol-dibutyrate-induced cytoskeletal remodeling in A7r5 smooth muscle cells. Exp Cell Res. 2002;280:64–74. - PubMed
-
- Linder S, Kopp P. Podosomes at a glance. J Cell Sc. 2005;118:2079–2082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

