Nitric oxide and peroxynitrite in health and disease
- PMID: 17237348
- PMCID: PMC2248324
- DOI: 10.1152/physrev.00029.2006
Nitric oxide and peroxynitrite in health and disease
Abstract
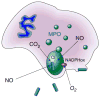
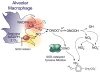
The discovery that mammalian cells have the ability to synthesize the free radical nitric oxide (NO) has stimulated an extraordinary impetus for scientific research in all the fields of biology and medicine. Since its early description as an endothelial-derived relaxing factor, NO has emerged as a fundamental signaling device regulating virtually every critical cellular function, as well as a potent mediator of cellular damage in a wide range of conditions. Recent evidence indicates that most of the cytotoxicity attributed to NO is rather due to peroxynitrite, produced from the diffusion-controlled reaction between NO and another free radical, the superoxide anion. Peroxynitrite interacts with lipids, DNA, and proteins via direct oxidative reactions or via indirect, radical-mediated mechanisms. These reactions trigger cellular responses ranging from subtle modulations of cell signaling to overwhelming oxidative injury, committing cells to necrosis or apoptosis. In vivo, peroxynitrite generation represents a crucial pathogenic mechanism in conditions such as stroke, myocardial infarction, chronic heart failure, diabetes, circulatory shock, chronic inflammatory diseases, cancer, and neurodegenerative disorders. Hence, novel pharmacological strategies aimed at removing peroxynitrite might represent powerful therapeutic tools in the future. Evidence supporting these novel roles of NO and peroxynitrite is presented in detail in this review.
Figures







Similar articles
-
Nitric oxide-derived oxidants with a focus on peroxynitrite: molecular targets, cellular responses and therapeutic implications.Curr Pharm Des. 2011 Dec;17(35):3905-32. doi: 10.2174/138161211798357719. Curr Pharm Des. 2011. PMID: 21933142 Review.
-
Cellular mechanisms of peroxynitrite-induced neuronal death.Brain Res Bull. 2017 Jul;133:4-11. doi: 10.1016/j.brainresbull.2017.05.008. Epub 2017 Jun 24. Brain Res Bull. 2017. PMID: 28655600 Review.
-
Role of redox signaling and poly (adenosine diphosphate-ribose) polymerase activation in vascular smooth muscle cell growth inhibition by nitric oxide and peroxynitrite.J Vasc Surg. 2008 Mar;47(3):599-607. doi: 10.1016/j.jvs.2007.11.006. J Vasc Surg. 2008. PMID: 18295111
-
The superoxide radical switch in the biology of nitric oxide and peroxynitrite.Physiol Rev. 2022 Oct 1;102(4):1881-1906. doi: 10.1152/physrev.00005.2022. Epub 2022 May 23. Physiol Rev. 2022. PMID: 35605280 Review.
-
Pathophysiological roles of peroxynitrite in circulatory shock.Shock. 2010 Sep;34 Suppl 1(0 1):4-14. doi: 10.1097/SHK.0b013e3181e7e9ba. Shock. 2010. PMID: 20523270 Free PMC article. Review.
Cited by
-
Membrane tubulovesicular extensions (cytonemes): secretory and adhesive cellular organelles.Cell Adh Migr. 2013 Mar-Apr;7(2):174-86. doi: 10.4161/cam.23130. Epub 2013 Jan 3. Cell Adh Migr. 2013. PMID: 23287580 Free PMC article. Review.
-
S-glutathionylation of ion channels: insights into the regulation of channel functions, thiol modification crosstalk, and mechanosensing.Antioxid Redox Signal. 2014 Feb 20;20(6):937-51. doi: 10.1089/ars.2013.5483. Epub 2013 Aug 20. Antioxid Redox Signal. 2014. PMID: 23834398 Free PMC article. Review.
-
Characterization of transgenic Gfrp knock-in mice: implications for tetrahydrobiopterin in modulation of normal tissue radiation responses.Antioxid Redox Signal. 2014 Mar 20;20(9):1436-46. doi: 10.1089/ars.2012.5025. Epub 2013 May 3. Antioxid Redox Signal. 2014. PMID: 23521531 Free PMC article.
-
Selective Acetamidine-Based Nitric Oxide Synthase Inhibitors: Synthesis, Docking, and Biological Studies.ACS Med Chem Lett. 2015 Apr 28;6(6):635-40. doi: 10.1021/acsmedchemlett.5b00149. eCollection 2015 Jun 11. ACS Med Chem Lett. 2015. PMID: 26101565 Free PMC article.
-
TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions.Cells. 2020 Sep 23;9(10):2145. doi: 10.3390/cells9102145. Cells. 2020. PMID: 32977412 Free PMC article. Review.
References
-
- Abdelkarim GE, Gertz K, Harms C, Katchanov J, Dirnagl U, Szabo C, Endres M. Protective effects of PJ34, a novel, potent inhibitor of poly(ADP-ribose) polymerase (PARP) in in vitro and in vivo models of stroke. Int J Mol Med. 2001;7:255–260. - PubMed
-
- Abe K, Aoki M, Kawagoe J, Yoshida T, Hattori A, Kogure K, Itoyama Y. Ischemic delayed neuronal death. A mitochondrial hypothesis. Stroke. 1995;26:1478–1489. - PubMed
-
- Abe K, Pan LH, Watanabe M, Kato T, Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199:152–154. - PubMed
-
- Abe K, Pan LH, Watanabe M, Konno H, Kato T, Itoyama Y. Upregulation of protein-tyrosine nitration in the anterior horn cells of amyotrophic lateral sclerosis. Neurol Res. 1997;19:124–128. - PubMed
-
- Abou-Mohamed G, Johnson JA, Jin L, El-Remessy AB, Do K, Kaesemeyer WH, Caldwell RB, Caldwell RW. Roles of superoxide, peroxynitrite, and protein kinase C in the development of tolerance to nitroglycerin. J Pharmacol Exp Ther. 2004;308:289–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

