The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells
- PMID: 17234767
- PMCID: PMC2679985
- DOI: 10.1158/0008-5472.CAN-06-2941
The inflammatory cytokine tumor necrosis factor-alpha generates an autocrine tumor-promoting network in epithelial ovarian cancer cells
Abstract
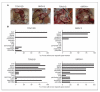
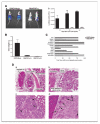
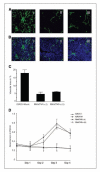
Constitutive expression of the inflammatory cytokine tumor necrosis factor-alpha (TNF-alpha) is characteristic of malignant ovarian surface epithelium. We investigated the hypothesis that this autocrine action of TNF-alpha generates and sustains a network of other mediators that promote peritoneal cancer growth and spread. When compared with two ovarian cancer cell lines that did not make TNF-alpha, constitutive production of TNF-alpha was associated with greater release of the chemokines CCL2 and CXCL12, the cytokines interleukin-6 (IL-6) and macrophage migration-inhibitory factor (MIF), and the angiogenic factor vascular endothelial growth factor (VEGF). TNF-alpha production was associated also with increased peritoneal dissemination when the ovarian cancer cells were xenografted. We next used RNA interference to generate stable knockdown of TNF-alpha in ovarian cancer cells. Production of CCL2, CXCL12, VEGF, IL-6, and MIF was decreased significantly in these cells compared with wild-type or mock-transfected cells, but in vitro growth rates were unaltered. Tumor growth and dissemination in vivo were significantly reduced when stable knockdown of TNF-alpha was achieved. Tumors derived from TNF-alpha knockdown cells were noninvasive and well circumscribed and showed high levels of apoptosis, even in the smallest deposits. This was reflected in reduced vascularization of TNF-alpha knockdown tumors. Furthermore, culture supernatants from such cells failed to stimulate endothelial cell growth in vitro. We conclude that autocrine production of TNF-alpha by ovarian cancer cells stimulates a constitutive network of other cytokines, angiogenic factors, and chemokines that may act in an autocrine/paracrine manner to promote colonization of the peritoneum and neovascularization of developing tumor deposits.
Figures


Similar articles
-
Impact of residual disease as a prognostic factor for survival in women with advanced epithelial ovarian cancer after primary surgery.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD015048. doi: 10.1002/14651858.CD015048.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161421 Free PMC article. Review.
-
A Blog-Based Study of Autistic Adults' Experiences of Aloneness and Connection and the Interplay with Well-Being: Corpus-Based and Thematic Analyses.Autism Adulthood. 2023 Dec 1;5(4):437-449. doi: 10.1089/aut.2022.0073. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116056 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Anti-angiogenic therapy for high-grade glioma.Cochrane Database Syst Rev. 2018 Nov 22;11(11):CD008218. doi: 10.1002/14651858.CD008218.pub4. Cochrane Database Syst Rev. 2018. PMID: 30480778 Free PMC article.
Cited by
-
Tumor progression locus 2 (Tpl2) kinase as a novel therapeutic target for cancer: double-sided effects of Tpl2 on cancer.Int J Mol Sci. 2015 Feb 25;16(3):4471-91. doi: 10.3390/ijms16034471. Int J Mol Sci. 2015. PMID: 25723737 Free PMC article. Review.
-
Increased tumor necrosis factor receptor 1 expression in human colorectal adenomas.World J Gastroenterol. 2012 Oct 14;18(38):5360-8. doi: 10.3748/wjg.v18.i38.5360. World J Gastroenterol. 2012. PMID: 23082052 Free PMC article.
-
In vivo single-cell CRISPR uncovers distinct TNF programmes in tumour evolution.Nature. 2024 Aug;632(8024):419-428. doi: 10.1038/s41586-024-07663-y. Epub 2024 Jul 17. Nature. 2024. PMID: 39020166 Free PMC article.
-
Gene expression profile analyze the molecular mechanism of CXCR7 regulating papillary thyroid carcinoma growth and metastasis.J Exp Clin Cancer Res. 2015 Feb 12;34(1):16. doi: 10.1186/s13046-015-0132-y. J Exp Clin Cancer Res. 2015. PMID: 25887589 Free PMC article.
-
Antitumor necrosis factor-α antibodies as a noveltherapy for hepatocellular carcinoma.Exp Ther Med. 2018 Aug;16(2):529-536. doi: 10.3892/etm.2018.6235. Epub 2018 May 30. Exp Ther Med. 2018. PMID: 30116311 Free PMC article.
References
-
- Moore R, Owens D, Stamp G, et al. Tumour necrosis factor-α deficient mice are resistant to skin carcinogenesis. Nat Med. 1999;5:828–31. - PubMed
-
- Arnott CH, Scott KA, Moore RJ, et al. Tumour necrosis factor-α mediates tumour promotion via a PKCα- AP-1-dependent pathway. Oncogene. 2002;21:4728–38. - PubMed
-
- Pikarsky E, Porat RM, Stein I, et al. NF-κB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:4461–6. - PubMed
-
- Szlosarek PW, Grimshaw MJ, Kulbe H, et al. Expression and regulation of tumor necrosis factor-α in normal and malignant ovarian epithelium. Mol Cancer Ther. 2006;5:382–90. - PubMed
-
- Szlosarek P, Balkwill F. Tumour necrosis factor-α: a potential target in the therapy of solid tumors. Lancet Oncol. 2003;4:565–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

