Avl9p, a member of a novel protein superfamily, functions in the late secretory pathway
- PMID: 17229886
- PMCID: PMC1838974
- DOI: 10.1091/mbc.e06-11-1035
Avl9p, a member of a novel protein superfamily, functions in the late secretory pathway
Abstract
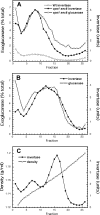
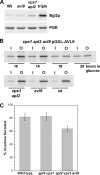
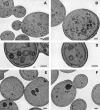
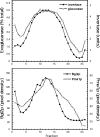
The branching of exocytic transport routes in both yeast and mammalian cells has complicated studies of the late secretory pathway, and the mechanisms involved in exocytic cargo sorting and exit from the Golgi and endosomes are not well understood. Because cargo can be sorted away from a blocked route and secreted by an alternate route, mutants defective in only one route do not exhibit a strong secretory phenotype and are therefore difficult to isolate. In a genetic screen designed to isolate such mutants, we identified a novel conserved protein, Avl9p, the absence of which conferred lethality in a vps1Delta apl2Delta strain background (lacking a dynamin and an adaptor-protein complex 1 subunit). Depletion of Avl9p in this strain resulted in secretory defects as well as accumulation of Golgi-like membranes. The triple mutant also had a depolarized actin cytoskeleton and defects in polarized secretion. Overexpression of Avl9p in wild-type cells resulted in vesicle accumulation and a post-Golgi defect in secretion. Phylogenetic analysis indicated evolutionary relationships between Avl9p and regulators of membrane traffic and actin function.
Figures


Similar articles
-
Yeast homologues of lethal giant larvae and type V myosin cooperate in the regulation of Rab-dependent vesicle clustering and polarized exocytosis.Mol Biol Cell. 2011 Mar 15;22(6):842-57. doi: 10.1091/mbc.E10-07-0570. Epub 2011 Jan 19. Mol Biol Cell. 2011. PMID: 21248204 Free PMC article.
-
A subset of yeast vacuolar protein sorting mutants is blocked in one branch of the exocytic pathway.J Cell Biol. 2002 Jan 21;156(2):271-85. doi: 10.1083/jcb.200109077. Epub 2002 Jan 21. J Cell Biol. 2002. PMID: 11807092 Free PMC article.
-
Exo70p mediates the secretion of specific exocytic vesicles at early stages of the cell cycle for polarized cell growth.J Cell Biol. 2007 Mar 12;176(6):771-7. doi: 10.1083/jcb.200606134. Epub 2007 Mar 5. J Cell Biol. 2007. PMID: 17339375 Free PMC article.
-
Membrane trafficking in the yeast Saccharomyces cerevisiae model.Int J Mol Sci. 2015 Jan 9;16(1):1509-25. doi: 10.3390/ijms16011509. Int J Mol Sci. 2015. PMID: 25584613 Free PMC article. Review.
-
An Overview of Protein Secretion in Yeast and Animal Cells.Methods Mol Biol. 2017;1662:1-17. doi: 10.1007/978-1-4939-7262-3_1. Methods Mol Biol. 2017. PMID: 28861813 Review.
Cited by
-
Circular RNA in Non-small Cell Lung Carcinoma: Identification of Targets and New Treatment Modalities.Cancer Genomics Proteomics. 2023 Dec;20(6suppl):646-668. doi: 10.21873/cgp.20413. Cancer Genomics Proteomics. 2023. PMID: 38035705 Free PMC article. Review.
-
Family-wide characterization of the DENN domain Rab GDP-GTP exchange factors.J Cell Biol. 2010 Oct 18;191(2):367-81. doi: 10.1083/jcb.201008051. Epub 2010 Oct 11. J Cell Biol. 2010. PMID: 20937701 Free PMC article.
-
A chemical genetic screen for modulators of exocytic transport identifies inhibitors of a transport mechanism linked to GTR2 function.Eukaryot Cell. 2010 Jan;9(1):116-26. doi: 10.1128/EC.00184-09. Epub 2009 Nov 6. Eukaryot Cell. 2010. PMID: 19897736 Free PMC article.
-
Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network.J Cell Biol. 2009 May 18;185(4):601-12. doi: 10.1083/jcb.200901145. Epub 2009 May 11. J Cell Biol. 2009. PMID: 19433450 Free PMC article.
-
The Connecdenn DENN domain: a GEF for Rab35 mediating cargo-specific exit from early endosomes.Mol Cell. 2010 Feb 12;37(3):370-82. doi: 10.1016/j.molcel.2009.12.037. Mol Cell. 2010. PMID: 20159556 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

