WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP)
- PMID: 17213309
- PMCID: PMC1783416
- DOI: 10.1073/pnas.0610275104
WIP is a chaperone for Wiskott-Aldrich syndrome protein (WASP)
Abstract
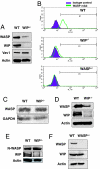
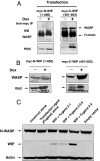
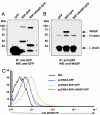
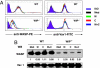
Wiskott-Aldrich syndrome protein (WASP) is in a complex with WASP-interacting protein (WIP). WASP levels, but not mRNA levels, were severely diminished in T cells from WIP(-/-) mice and were increased by introduction of WIP in these cells. The WASP binding domain of WIP was shown to protect WASP from degradation by calpain in vitro. Treatment with the proteasome inhibitors MG132 and bortezomib increased WASP levels in T cells from WIP(-/-) mice and in T and B lymphocytes from two WAS patients with missense mutations (R86H and T45M) that disrupt WIP binding. The calpain inhibitor calpeptin increased WASP levels in activated T and B cells from the WASP patients, but not in primary T cells from the patients or from WIP(-/-) mice. Despite its ability to increase WASP levels proteasome inhibition did not correct the impaired IL-2 gene expression and low F-actin content in T cells from the R86H WAS patient. These results demonstrate that WIP stabilizes WASP and suggest that it may also be important for its function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A peptide derived from the Wiskott-Aldrich syndrome (WAS) protein-interacting protein (WIP) restores WAS protein level and actin cytoskeleton reorganization in lymphocytes from patients with WAS mutations that disrupt WIP binding.J Allergy Clin Immunol. 2011 Apr;127(4):998-1005.e1-2. doi: 10.1016/j.jaci.2011.01.015. Epub 2011 Mar 3. J Allergy Clin Immunol. 2011. PMID: 21376381 Free PMC article.
-
T-cell receptor ligation causes Wiskott-Aldrich syndrome protein degradation and F-actin assembly downregulation.J Allergy Clin Immunol. 2013 Sep;132(3):648-655.e1. doi: 10.1016/j.jaci.2013.03.046. Epub 2013 May 16. J Allergy Clin Immunol. 2013. PMID: 23684068
-
WIP, a protein associated with wiskott-aldrich syndrome protein, induces actin polymerization and redistribution in lymphoid cells.Proc Natl Acad Sci U S A. 1997 Dec 23;94(26):14671-6. doi: 10.1073/pnas.94.26.14671. Proc Natl Acad Sci U S A. 1997. PMID: 9405671 Free PMC article.
-
WASP-WIP complex in the molecular pathogenesis of Wiskott-Aldrich syndrome.Pediatr Int. 2016 Jan;58(1):4-7. doi: 10.1111/ped.12819. Epub 2015 Dec 5. Pediatr Int. 2016. PMID: 26331277 Review.
-
Recent advances in the biology of WASP and WIP.Immunol Res. 2009;44(1-3):99-111. doi: 10.1007/s12026-008-8086-1. Immunol Res. 2009. PMID: 19018480 Review.
Cited by
-
FLI1 Induces Megakaryopoiesis Gene Expression Through WAS/WIP-Dependent and Independent Mechanisms; Implications for Wiskott-Aldrich Syndrome.Front Immunol. 2021 Feb 26;12:607836. doi: 10.3389/fimmu.2021.607836. eCollection 2021. Front Immunol. 2021. PMID: 33717090 Free PMC article.
-
4D non-uniformly sampled HCBCACON and ¹J(NCα)-selective HCBCANCO experiments for the sequential assignment and chemical shift analysis of intrinsically disordered proteins.J Biomol NMR. 2012 Jun;53(2):139-48. doi: 10.1007/s10858-012-9631-8. Epub 2012 May 13. J Biomol NMR. 2012. PMID: 22580891
-
Ubiquitylation-dependent negative regulation of WASp is essential for actin cytoskeleton dynamics.Mol Cell Biol. 2012 Aug;32(15):3153-63. doi: 10.1128/MCB.00161-12. Epub 2012 Jun 4. Mol Cell Biol. 2012. PMID: 22665495 Free PMC article.
-
Actin engine in immunological synapse.Immune Netw. 2012 Jun;12(3):71-83. doi: 10.4110/in.2012.12.3.71. Epub 2012 Jun 30. Immune Netw. 2012. PMID: 22916042 Free PMC article.
-
A peptide derived from the Wiskott-Aldrich syndrome (WAS) protein-interacting protein (WIP) restores WAS protein level and actin cytoskeleton reorganization in lymphocytes from patients with WAS mutations that disrupt WIP binding.J Allergy Clin Immunol. 2011 Apr;127(4):998-1005.e1-2. doi: 10.1016/j.jaci.2011.01.015. Epub 2011 Mar 3. J Allergy Clin Immunol. 2011. PMID: 21376381 Free PMC article.
References
-
- Derry JMJ, Ochs HD, Francke U. Cell. 1994;78:635–644. - PubMed
-
- Cannon JL, Labno CM, Bosco G, Seth A, McGavin MH, Siminovitch KA, Rosen MK, Burkhardt JK. Immunity. 2001;15:249–259. - PubMed
-
- Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N, Geha RS. Mol Cell. 2002;10:1269–1281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

