Propagation of the cardiac impulse in the diabetic rat heart: reduced conduction reserve
- PMID: 17185336
- PMCID: PMC2075555
- DOI: 10.1113/jphysiol.2006.123729
Propagation of the cardiac impulse in the diabetic rat heart: reduced conduction reserve
Abstract
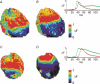
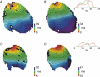
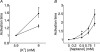
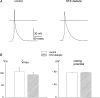
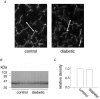
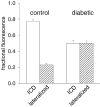
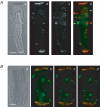
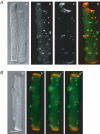
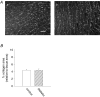
Diabetes mellitus is a growing epidemic with severe cardiovascular complications. Although much is known about mechanical and electrical cardiac dysfunction in diabetes, few studies have investigated propagation of the electrical signal in the diabetic heart and the associated changes in intercellular gap junctions. This study was designed to investigate these issues, using hearts from control and diabetic rats. Diabetic conditions were induced by streptozotocin (STZ), given i.v. 7-14 days before experiments. Optical mapping with the voltage-sensitive dye di-4-ANEPPS, using hearts perfused on a Langendorff apparatus, showed little change in baseline conduction velocity in diabetic hearts, reflecting the large reserve of function. However, both the gap junction uncoupler heptanol (0.5-1 mM) and elevated potassium (9 mM, to reduce cell excitability) produced a significantly greater slowing of impulse propagation in diabetic hearts than in controls. The maximal action potential upstroke velocity (an index of the sodium current) and resting potential was similar in single ventricular myocytes from control and diabetic rats, suggesting similar electrical excitability. Immunoblotting of connexin 43 (Cx43), a major gap junction component, showed no change in total expression. However, immunofluorescence labelling of Cx43 showed a significant redistribution, apparent as enhanced Cx43 lateralization. This was quantified and found to be significantly larger than in control myocytes. Labelling of two other gap junction proteins, N-cadherin and beta-catenin, showed a (partial) loss of co-localization with Cx43, indicating that enhancement of lateralized Cx43 is associated with non-functional gap junctions. In conclusion, conduction reserve is smaller in the diabetic heart, priming it for impaired conduction upon further challenges. This can desynchronize contraction and contribute to arrhythmogenesis.
Figures

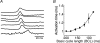
Similar articles
-
Sex-dependent impairment of cardiac action potential conduction in type 1 diabetic rats.Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1442-50. doi: 10.1152/ajpheart.01150.2008. Epub 2009 Mar 13. Am J Physiol Heart Circ Physiol. 2009. PMID: 19286947
-
Reduced conduction reserve in the diabetic rat heart: role of iPLA2 activation in the response to ischemia.Am J Physiol Heart Circ Physiol. 2011 Jan;300(1):H326-34. doi: 10.1152/ajpheart.00743.2010. Epub 2010 Oct 29. Am J Physiol Heart Circ Physiol. 2011. PMID: 21037228
-
The rate and anisotropy of impulse propagation in the postnatal terminal crest are correlated with remodeling of Cx43 gap junction pattern.Cardiovasc Res. 2000 Jan 14;45(2):379-87. doi: 10.1016/s0008-6363(99)00363-6. Cardiovasc Res. 2000. PMID: 10728358
-
Anisotropic activation spread in heart cell monolayers assessed by high-resolution optical mapping. Role of tissue discontinuities.Circ Res. 1996 Jul;79(1):115-27. doi: 10.1161/01.res.79.1.115. Circ Res. 1996. PMID: 8925559 Review.
-
[Remodeling of cardiac gap junctions and arrhythmias].Sheng Li Xue Bao. 2011 Dec 25;63(6):586-92. Sheng Li Xue Bao. 2011. PMID: 22193455 Review. Chinese.
Cited by
-
A simulation study of cellular hypertrophy and connexin lateralization in cardiac tissue.Biophys J. 2010 Nov 3;99(9):2821-30. doi: 10.1016/j.bpj.2010.09.010. Biophys J. 2010. PMID: 21044579 Free PMC article.
-
Advanced Glycation End Product (AGE)-AGE Receptor (RAGE) System Upregulated Connexin43 Expression in Rat Cardiomyocytes via PKC and Erk MAPK Pathways.Int J Mol Sci. 2013 Jan 24;14(2):2242-57. doi: 10.3390/ijms14022242. Int J Mol Sci. 2013. PMID: 23348924 Free PMC article.
-
Novel Methods for High-resolution Assessment of Cardiac Action Potential Repolarization.Biomed Signal Process Control. 2019 May;51:30-41. doi: 10.1016/j.bspc.2019.02.003. Epub 2019 Feb 18. Biomed Signal Process Control. 2019. PMID: 31938034 Free PMC article.
-
Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection.Pharmacol Ther. 2015 Sep;153:90-106. doi: 10.1016/j.pharmthera.2015.06.005. Epub 2015 Jun 11. Pharmacol Ther. 2015. PMID: 26073311 Free PMC article. Review.
-
Connexins in Cardiovascular and Neurovascular Health and Disease: Pharmacological Implications.Pharmacol Rev. 2017 Oct;69(4):396-478. doi: 10.1124/pr.115.012062. Pharmacol Rev. 2017. PMID: 28931622 Free PMC article. Review.
References
-
- Abo K, Ishida Y, Yoshida R, Hozumi T, Ueno H, Shiotani H, Matsunaga K, Kazumi T. Torsade de pointes in NIDDM with long QT intervals. Diabetes Care. 1996;19:1010. - PubMed
-
- Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2005;95:717–725. - PubMed
-
- Baker LC, Wolk R, Choi BR, Watkins S, Plan P, Shah A, Salama G. Effects of mechanical uncouplers, diacetyl monoxime, and cytochalasin-D on the electrophysiology of perfused mouse hearts. Am J Physiol Heart Circ Physiol. 2004;287:H1771–H1779. - PubMed
-
- Bollano E, Omerovic E, Svensson H, Waagstein F, Fu M. Cardiac remodelling rather than disturbed myocardial energy metabolism is associated with cardiac dysfunction in diabetic rats. Int J Cardiol. 2006;114:195–201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

