COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase
- PMID: 17183367
- PMCID: PMC1783443
- DOI: 10.1038/sj.emboj.7601489
COMMD1 promotes the ubiquitination of NF-kappaB subunits through a cullin-containing ubiquitin ligase
Abstract
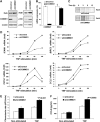
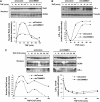
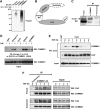
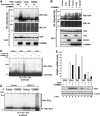
NF-kappaB is a pleiotropic transcription factor involved in multiple processes, including inflammation and oncogenesis. We have previously reported that COMMD1 represses kappaB-dependent transcription by negatively regulating NF-kappaB-chromatin interactions. Recently, ubiquitination of NF-kappaB subunits has been similarly implicated in the control of NF-kappaB recruitment to chromatin. We report here that COMMD1 accelerates the ubiquitination and degradation of NF-kappaB subunits through its interaction with a multimeric ubiquitin ligase containing Elongins B and C, Cul2 and SOCS1 (ECS(SOCS1)). COMMD1-deficient cells demonstrate stabilization of RelA, greater nuclear accumulation of RelA after TNF stimulation, de-repression of several kappaB-responsive genes, and enhanced NF-kappaB-mediated cellular responses. COMMD1 binds to Cul2 in a stimulus-dependent manner and serves to facilitate substrate binding to the ligase by stabilizing the interaction between SOCS1 and RelA. Our data uncover that ubiquitination and degradation of NF-kappaB subunits by this COMMD1-containing ubiquitin ligase is a novel and critical mechanism of regulation of NF-kappaB-mediated transcription.
Figures

Similar articles
-
CSN-associated USP48 confers stability to nuclear NF-κB/RelA by trimming K48-linked Ub-chains.Biochim Biophys Acta. 2015 Feb;1853(2):453-69. doi: 10.1016/j.bbamcr.2014.11.028. Epub 2014 Dec 5. Biochim Biophys Acta. 2015. PMID: 25486460
-
GCN5 is a required cofactor for a ubiquitin ligase that targets NF-kappaB/RelA.Genes Dev. 2009 Apr 1;23(7):849-61. doi: 10.1101/gad.1748409. Genes Dev. 2009. PMID: 19339690 Free PMC article.
-
Regulation of NF-kappaB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA.Mol Cell. 2003 Dec;12(6):1413-26. doi: 10.1016/s1097-2765(03)00490-8. Mol Cell. 2003. PMID: 14690596
-
COMMD proteins and the control of the NF kappa B pathway.Cell Cycle. 2007 Mar 15;6(6):672-6. doi: 10.4161/cc.6.6.3989. Epub 2007 Mar 7. Cell Cycle. 2007. PMID: 17361106 Free PMC article. Review.
-
Linear ubiquitination: a novel NF-κB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex.Endocr J. 2012;59(8):641-52. doi: 10.1507/endocrj.ej12-0148. Epub 2012 May 19. Endocr J. 2012. PMID: 22673407 Review.
Cited by
-
Structural organization of the retriever-CCC endosomal recycling complex.Nat Struct Mol Biol. 2024 Jun;31(6):910-924. doi: 10.1038/s41594-023-01184-4. Epub 2023 Dec 7. Nat Struct Mol Biol. 2024. PMID: 38062209 Free PMC article.
-
Regulation of nuclear factor κB (NF-κB) transcriptional activity via p65 acetylation by the chaperonin containing TCP1 (CCT).PLoS One. 2012;7(7):e42020. doi: 10.1371/journal.pone.0042020. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860050 Free PMC article.
-
PPARγ is an E3 ligase that induces the degradation of NFκB/p65.Nat Commun. 2012;3:1300. doi: 10.1038/ncomms2270. Nat Commun. 2012. PMID: 23250430
-
Nuclear COMMD1 Is Associated with Cisplatin Sensitivity in Ovarian Cancer.PLoS One. 2016 Oct 27;11(10):e0165385. doi: 10.1371/journal.pone.0165385. eCollection 2016. PLoS One. 2016. PMID: 27788210 Free PMC article.
-
An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics.Emerg Microbes Infect. 2020 Dec;9(1):2333-2347. doi: 10.1080/22221751.2020.1826361. Emerg Microbes Infect. 2020. PMID: 32954948 Free PMC article.
References
-
- Burstein E, Hoberg JE, Wilkinson AS, Rumble JM, Csomos RA, Komarck CM, Maine GN, Wilkinson JC, Mayo MW, Duckett CS (2005) COMMD proteins: a novel family of structural and functional homologs of MURR1. J Biol Chem 280: 22222–22232 - PubMed
-
- Chen L, Fischle W, Verdin E, Greene WC (2001) Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293: 1653–1657 - PubMed
-
- Chen Z, Hagler J, Palombella VJ, Melandri F, Scherer D, Ballard D, Maniatis T (1995) Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin–proteasome pathway. Genes Dev 9: 1586–1597 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

