Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts
- PMID: 17151134
- PMCID: PMC1797553
- DOI: 10.1128/JVI.02110-06
Herpes simplex virus type 2 (HSV-2) establishes latent infection in a different population of ganglionic neurons than HSV-1: role of latency-associated transcripts
Abstract
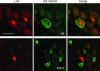
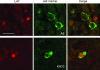
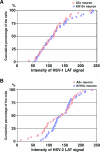
Herpes simplex virus type 1 (HSV-1) and HSV-2 cause very similar acute infections but differ in their abilities to reactivate from trigeminal and dorsal root ganglia. To investigate differences in patterns of viral infection, we colabeled murine sensory ganglia for evidence of HSV infection and for the sensory neuron marker A5 or KH10. During acute infection, 7 to 10% of HSV-1 or HSV-2 antigen-positive neurons were A5 positive and 13 to 16% were KH10 positive, suggesting that both viruses reach each type of neuron in a manner proportional to their representation in uninfected ganglia. In murine trigeminal ganglia harvested during HSV latency, 25% of HSV-1 latency-associated transcript (LAT)- and 4% of HSV-2 LAT-expressing neurons were A5 positive, while 12% of HSV-1 LAT- and 42% of HSV-2 LAT-expressing neurons were KH10 positive. A similar difference was observed in murine dorsal root ganglia. These differences could not be attributed to differences in LAT expression levels in A5- versus KH10-positive neurons. Thus, HSV-1 demonstrated a preference for the establishment of latency in A5-positive neurons, while HSV-2 demonstrated a preference for the establishment of latency in KH10-positive neurons. A chimeric HSV-2 mutant that expresses the HSV-1 LAT exhibited an HSV-1 phenotype, preferentially establishing latency in A5-positive neurons. These data imply that the HSV-1 and HSV-2 LAT regions influence the ability of virus to establish latency in different neuronal subtypes. That the same chimeric virus has a characteristic HSV-1 reactivation phenotype further suggests that LAT-influenced establishment of latency in specific neuronal subtypes could be an important part of the mechanism by which LAT influences viral reactivation phenotypes.
Figures
Similar articles
-
Investigation of the mechanism by which herpes simplex virus type 1 LAT sequences modulate preferential establishment of latent infection in mouse trigeminal ganglia.J Virol. 2009 Aug;83(16):7873-82. doi: 10.1128/JVI.00043-09. Epub 2009 Jun 3. J Virol. 2009. PMID: 19493993 Free PMC article.
-
Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT.J Virol. 2009 Oct;83(19):10007-15. doi: 10.1128/JVI.00559-09. Epub 2009 Jul 29. J Virol. 2009. PMID: 19641003 Free PMC article.
-
Different mechanisms regulate productive herpes simplex virus 1 (HSV-1) and HSV-2 infections in adult trigeminal neurons.J Virol. 2013 Jun;87(11):6512-6. doi: 10.1128/JVI.00383-13. Epub 2013 Mar 20. J Virol. 2013. PMID: 23514893 Free PMC article.
-
A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation.J Gen Virol. 2015 Jul;96(Pt 7):1581-602. doi: 10.1099/vir.0.000128. Epub 2015 Mar 20. J Gen Virol. 2015. PMID: 25794504 Free PMC article. Review.
-
The latency-associated gene of herpes simplex virus type 1 (HSV-1) interferes with superinfection by HSV-1.J Neurovirol. 2002 Dec;8 Suppl 2:97-102. doi: 10.1080/13550280290167920. J Neurovirol. 2002. PMID: 12491159 Review.
Cited by
-
Evasion of early antiviral responses by herpes simplex viruses.Mediators Inflamm. 2015;2015:593757. doi: 10.1155/2015/593757. Epub 2015 Mar 30. Mediators Inflamm. 2015. PMID: 25918478 Free PMC article. Review.
-
Disease-modifying rdHSV-CA8* non-opioid analgesic gene therapy treats chronic osteoarthritis pain by activating Kv7 voltage-gated potassium channels.Front Mol Neurosci. 2024 Jul 17;17:1416148. doi: 10.3389/fnmol.2024.1416148. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39086927 Free PMC article.
-
Intimate Relationship Between Stress and Human Alpha‑Herpes Virus 1 (HSV‑1) Reactivation from Latency.Curr Clin Microbiol Rep. 2023 Dec;10(4):236-245. doi: 10.1007/s40588-023-00202-9. Epub 2023 Jul 27. Curr Clin Microbiol Rep. 2023. PMID: 38173564 Free PMC article.
-
Galectins as potential therapeutic targets in STIs in the female genital tract.Nat Rev Urol. 2022 Apr;19(4):240-252. doi: 10.1038/s41585-021-00562-1. Epub 2022 Feb 1. Nat Rev Urol. 2022. PMID: 35105978 Review.
-
CGRP inhibits human Langerhans cells infection with HSV by differentially modulating specific HSV-1 and HSV-2 entry mechanisms.Mucosal Immunol. 2022 Apr;15(4):762-771. doi: 10.1038/s41385-022-00521-y. Epub 2022 May 13. Mucosal Immunol. 2022. PMID: 35562558
References
-
- Bloom, D. C. 2004. HSV LAT and neuronal survival. Int. Rev. Immunol. 23:187-198. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

