Galectin-3 associates with the primary cilium and modulates cyst growth in congenital polycystic kidney disease
- PMID: 17148658
- PMCID: PMC1762475
- DOI: 10.2353/ajpath.2006.060245
Galectin-3 associates with the primary cilium and modulates cyst growth in congenital polycystic kidney disease
Abstract
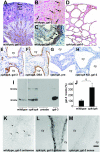
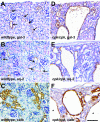
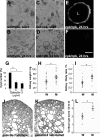
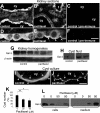
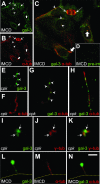
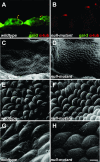
Several lines of evidence implicate the beta-galactoside-binding lectin galectin-3 in development and pathological processes in renal collecting ducts: galectin-3 is expressed in the ureteric bud/collecting duct lineage during nephrogenesis, modulates collecting duct growth/differentiation in vitro, and is expressed in human autosomal recessive polycystic kidney disease in cyst epithelia, almost all of which arise from collecting ducts. Moreover, exogenous galectin-3 restricts growth of cysts generated by Madin-Darby canine kidney collecting duct-derived cells in three-dimensional culture in collagen. Using the cpk mouse model of recessively inherited polycystic kidney disease, we observed widespread galectin-3 mRNA and protein in cyst epithelia. Exogenous galectin-3 reduced cyst formation in suspension culture, and mice-null mutant for galectin-3 had more extensive renal cysts in vivo. Galectin-3 was also detected for the first time in the centrosome/primary cilium, which has been implicated in diverse polycystic kidney disease. Cilia structure/number appeared normal in galectin-3-null mutants. Finally, paclitaxel, a therapy that retards polycystic kidney disease in cpk mice, increased extracellular galectin-3, in which the lectin could potentially interact with cilia. These data raise the possibility that galectin-3 may act as a natural brake on cystogenesis in cpk mice, perhaps via ciliary roles.
Figures
Similar articles
-
The P2X7 ATP receptor modulates renal cyst development in vitro.Biochem Biophys Res Commun. 2004 Sep 17;322(2):434-9. doi: 10.1016/j.bbrc.2004.07.148. Biochem Biophys Res Commun. 2004. PMID: 15325248
-
P2X(7) receptors are expressed during mouse nephrogenesis and in collecting duct cysts of the cpk/cpk mouse.Exp Nephrol. 2002;10(1):34-42. doi: 10.1159/000049896. Exp Nephrol. 2002. PMID: 11803203
-
The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia.J Am Soc Nephrol. 2002 Oct;13(10):2508-16. doi: 10.1097/01.asn.0000029587.47950.25. J Am Soc Nephrol. 2002. PMID: 12239239
-
Polycystic kidney disease and the renal cilium.Nephrology (Carlton). 2007 Dec;12(6):559-64. doi: 10.1111/j.1440-1797.2007.00869.x. Nephrology (Carlton). 2007. PMID: 17995581 Review.
-
Polycystic kidney disease: the cilium as a common pathway in cystogenesis.Curr Opin Pediatr. 2004 Apr;16(2):171-6. doi: 10.1097/00008480-200404000-00010. Curr Opin Pediatr. 2004. PMID: 15021197 Review.
Cited by
-
Differential expression of renal proteins in a rodent model of Meckel syndrome.Nephron Exp Nephrol. 2011;117(2):e31-8. doi: 10.1159/000319722. Epub 2010 Aug 6. Nephron Exp Nephrol. 2011. PMID: 20693816 Free PMC article.
-
Expression of Fraser syndrome genes in normal and polycystic murine kidneys.Pediatr Nephrol. 2012 Jun;27(6):991-8. doi: 10.1007/s00467-012-2100-5. Epub 2011 Oct 13. Pediatr Nephrol. 2012. PMID: 21993971 Free PMC article.
-
Modified citrus pectin reduces galectin-3 expression and disease severity in experimental acute kidney injury.PLoS One. 2011 Apr 8;6(4):e18683. doi: 10.1371/journal.pone.0018683. PLoS One. 2011. PMID: 21494626 Free PMC article.
-
Galectin-3 in Kidney Diseases: From an Old Protein to a New Therapeutic Target.Int J Mol Sci. 2022 Mar 14;23(6):3124. doi: 10.3390/ijms23063124. Int J Mol Sci. 2022. PMID: 35328545 Free PMC article. Review.
-
Galectin-3, a novel centrosome-associated protein, required for epithelial morphogenesis.Mol Biol Cell. 2010 Jan 15;21(2):219-31. doi: 10.1091/mbc.e09-03-0193. Epub 2009 Nov 18. Mol Biol Cell. 2010. PMID: 19923323 Free PMC article.
References
-
- Barondes SH, Castronovo V, Cooper DN, Cummings RD, Drickamer K, Feizi T, Gitt MA, Hirabayashi J, Hughes C, Kasai K, Leffler H, Liu FT, Lotan R, Mercurio AM, Monsigny M, Pillai S, Poirier F, Raz A, Rigby PWJ, Rini JM. Galectins: a family of animal β-galactoside binding lectins. Cell. 1994;76:597–598. - PubMed
-
- Leffler H, Barondes SH. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian β-galactosides. J Biol Chem. 1986;261:10119–10126. - PubMed
-
- Winyard PJ, Bao Q, Hughes RC, Woolf AS. Epithelial galectin-3 during human nephrogenesis and childhood cystic diseases. J Am Soc Nephrol. 1997;8:1647–1657. - PubMed
-
- Bichara M, Attmane-Elakeb A, Brown D, Essig M, Karim Z, Muffat-Joly M, Micheli L, Eude-le-Parco I, Cluzeaud F, Peuchmaur M, Bonvalet JP, Poirier F, Farman N. Exploring the role of galectin-3 in kidney function: a genetic approach. Glycobiology. 2006;16:36–45. - PubMed
-
- Bullock SL, Johnson TM, Bao Q, Hughes RC, Winyard PJ, Woolf AS. Galectin-3 modulates ureteric bud branching in organ culture of the developing mouse kidney. J Am Soc Nephrol. 2001;12:515–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

