Cloning, expression, crystallization and preliminary crystallographic analysis of a pentapeptide-repeat protein (Rfr23) from the bacterium Cyanothece 51142
- PMID: 17142909
- PMCID: PMC2225376
- DOI: 10.1107/S174430910604663X
Cloning, expression, crystallization and preliminary crystallographic analysis of a pentapeptide-repeat protein (Rfr23) from the bacterium Cyanothece 51142
Abstract
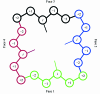
A unique feature of cyanobacteria genomes is the abundance of genes that code for hypothetical proteins containing tandem pentapeptide repeats approximately described by the consensus motif A(N/D)LXX. To date, the structures of two pentapeptide-repeat proteins (PRPs) have been determined, with the tandem pentapeptide-repeat sequences observed to adopt a novel type of right-handed quadrilateral beta-helix, or Rfr-fold, in both structures. One structure, Mycobacterium tuberculosis MfpA, is a 183-residue protein that contains 30 consecutive pentapeptide repeats and appears to offer antibiotic resistance by acting as a DNA mimic. The other structure, Cyanothece 51142 Rfr32, is a 167-residue protein that contains 21 consecutive pentapeptide repeats. The function of Rfr32, like the other 35 hypothetical PRPs identified in the genome of Cyanothece, is unknown. In an effort to understand the role of PRPs in cyanobacteria and to better characterize the structural properties of Rfr-folds with different amino-acid sequences, a second PRP from Cyanothece 51142, Rfr23, has been cloned, expressed and purified. Selenomethione-substituted protein was crystallized by vapor diffusion in hanging drops. Nearly complete SAD and native diffraction data sets were collected from these crystals to 2.5 and 2.1 A resolution, respectively, using synchrotron radiation. The crystals belonged to space group I4(1), with unit-cell parameters a = b = 106.61, c = 53.37 A, and one molecule per asymmetric unit. Preliminary analysis of the electron-density map from the SAD data shows that Rfr23 contains an Rfr-fold.
Figures

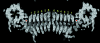
Similar articles
-
Insights into the structural variation between pentapeptide repeat proteins--crystal structure of Rfr23 from Cyanothece 51142.J Struct Biol. 2008 Apr;162(1):184-92. doi: 10.1016/j.jsb.2007.11.008. Epub 2007 Nov 28. J Struct Biol. 2008. PMID: 18158251
-
Characterization of two potentially universal turn motifs that shape the repeated five-residues fold--crystal structure of a lumenal pentapeptide repeat protein from Cyanothece 51142.Protein Sci. 2006 Nov;15(11):2579-95. doi: 10.1110/ps.062407506. Protein Sci. 2006. PMID: 17075135 Free PMC article.
-
Crystallization and crystallographic analysis of branching enzymes from Cyanothece sp. ATCC 51142.Acta Crystallogr F Struct Biol Commun. 2015 Aug;71(Pt 8):1109-13. doi: 10.1107/S2053230X1501198X. Epub 2015 Jul 29. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26249708 Free PMC article.
-
Structural dynamics of pentapeptide repeat proteins.Proteins. 2020 Nov;88(11):1493-1512. doi: 10.1002/prot.25969. Epub 2020 Jul 11. Proteins. 2020. PMID: 32548861
-
Current Understanding of the Structure and Function of Pentapeptide Repeat Proteins.Biomolecules. 2021 Apr 26;11(5):638. doi: 10.3390/biom11050638. Biomolecules. 2021. PMID: 33925937 Free PMC article. Review.
Cited by
-
Botulinum Neurotoxin Serotype A Recognizes Its Protein Receptor SV2 by a Different Mechanism than Botulinum Neurotoxin B Synaptotagmin.Toxins (Basel). 2016 May 17;8(5):154. doi: 10.3390/toxins8050154. Toxins (Basel). 2016. PMID: 27196927 Free PMC article.
References
-
- Doublié, S. (1997). Methods Enzymol.276, 523–530. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

