Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes
- PMID: 17130288
- PMCID: PMC2064671
- DOI: 10.1083/jcb.200606113
Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes
Erratum in
- J Cell Biol. 2006 Dec 18;175(6):1043
Abstract
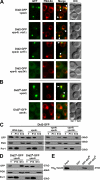
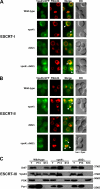
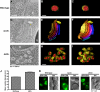
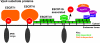
The sorting of transmembrane cargo proteins into the lumenal vesicles of multivesicular bodies (MVBs) depends on the recruitment of endosomal sorting complexes required for transport (ESCRTs) to the cytosolic face of endosomal membranes. The subsequent dissociation of ESCRT complexes from endosomes requires Vps4, a member of the AAA family of adenosine triphosphatases. We show that Did2 directs Vps4 activity to the dissociation of ESCRT-III but has no role in the dissociation of ESCRT-I or -II. Surprisingly, vesicle budding into the endosome lumen occurs in the absence of Did2 function even though Did2 is required for the efficient sorting of MVB cargo proteins into lumenal vesicles. This uncoupling of MVB cargo sorting and lumenal vesicle formation suggests that the Vps4-mediated dissociation of ESCRT-III is an essential step in the sorting of cargo proteins into MVB vesicles but is not a prerequisite for the budding of vesicles into the endosome lumen.
Figures

Similar articles
-
A concentric circle model of multivesicular body cargo sorting.EMBO Rep. 2007 Jul;8(7):644-50. doi: 10.1038/sj.embor.7401004. EMBO Rep. 2007. PMID: 17603537 Free PMC article. Review.
-
Regulators of Vps4 ATPase activity at endosomes differentially influence the size and rate of formation of intralumenal vesicles.Mol Biol Cell. 2010 Mar 15;21(6):1023-32. doi: 10.1091/mbc.e09-09-0776. Epub 2010 Jan 20. Mol Biol Cell. 2010. PMID: 20089837 Free PMC article.
-
Bro1 is an endosome-associated protein that functions in the MVB pathway in Saccharomyces cerevisiae.J Cell Sci. 2003 May 15;116(Pt 10):1893-903. doi: 10.1242/jcs.00395. Epub 2003 Mar 18. J Cell Sci. 2003. PMID: 12668726
-
Vps4 regulates a subset of protein interactions at the multivesicular endosome.FEBS J. 2007 Apr;274(8):1894-907. doi: 10.1111/j.1742-4658.2007.05736.x. Epub 2007 Mar 5. FEBS J. 2007. PMID: 17408385
-
Regulation of Vps4 ATPase activity by ESCRT-III.Biochem Soc Trans. 2009 Feb;37(Pt 1):143-5. doi: 10.1042/BST0370143. Biochem Soc Trans. 2009. PMID: 19143619 Free PMC article. Review.
Cited by
-
Relief of autoinhibition enhances Vta1 activation of Vps4 via the Vps4 stimulatory element.J Biol Chem. 2013 Sep 6;288(36):26147-26156. doi: 10.1074/jbc.M113.494112. Epub 2013 Jul 23. J Biol Chem. 2013. PMID: 23880759 Free PMC article.
-
The ESCRT complexes.Crit Rev Biochem Mol Biol. 2010 Dec;45(6):463-87. doi: 10.3109/10409238.2010.502516. Epub 2010 Jul 23. Crit Rev Biochem Mol Biol. 2010. PMID: 20653365 Free PMC article. Review.
-
A concentric circle model of multivesicular body cargo sorting.EMBO Rep. 2007 Jul;8(7):644-50. doi: 10.1038/sj.embor.7401004. EMBO Rep. 2007. PMID: 17603537 Free PMC article. Review.
-
Molecular mechanism of multivesicular body biogenesis by ESCRT complexes.Nature. 2010 Apr 8;464(7290):864-9. doi: 10.1038/nature08849. Epub 2010 Mar 21. Nature. 2010. PMID: 20305637 Free PMC article.
-
Computational model of membrane fission catalyzed by ESCRT-III.PLoS Comput Biol. 2009 Nov;5(11):e1000575. doi: 10.1371/journal.pcbi.1000575. Epub 2009 Nov 20. PLoS Comput Biol. 2009. PMID: 19936052 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

