Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells
- PMID: 17108037
- PMCID: PMC1797501
- DOI: 10.1128/JVI.01861-06
Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells
Abstract
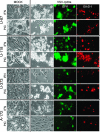
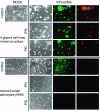
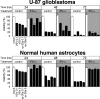
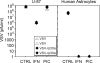
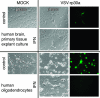
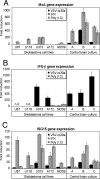
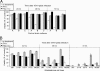
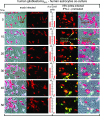
With little improvement in the poor prognosis for humans with high-grade glioma brain tumors, alternative therapeutic strategies are needed. As such, selective replication-competent oncolytic viruses may be useful as a potential treatment modality. Here we test the hypothesis that defects in the interferon (IFN) pathway could be exploited to enhance the selective oncolytic profile of vesicular stomatitis virus (VSV) in glioblastoma cells. Two green fluorescent protein-expressing VSV strains, recombinant VSV and the glioma-adapted recombinant VSV-rp30a, were used to study infection of a variety of human glioblastoma cell lines compared to a panel of control cells, including normal human astrocytes, oligodendrocyte precursor cells, and primary explant cultures from human brain tissue. Infection rate, cell viability, viral replication, and IFN-alpha/beta-related gene expression were compared in the absence and presence of IFN-alpha or polyriboinosinic polyribocytidylic acid [poly(I:C)], a synthetic inducer of the IFN-alpha/beta pathway. Both VSV strains caused rapid and total infection and death of all tumor cell lines tested. To a lesser degree, normal cells were also subject to VSV infection. In contrast, IFN-alpha or poly(I:C) completely attenuated the infection of all primary control brain cells, whereas most glioblastoma cell lines treated with IFN-alpha or poly(I:C) showed little or no sign of protection and were killed by VSV. Together, our results demonstrate that activation of the interferon pathway protects normal human brain cells from VSV infection while maintaining the vulnerability of human glioblastoma cells to viral destruction.
Figures

Similar articles
-
Interferon Beta and Interferon Alpha 2a Differentially Protect Head and Neck Cancer Cells from Vesicular Stomatitis Virus-Induced Oncolysis.J Virol. 2015 Aug;89(15):7944-54. doi: 10.1128/JVI.00757-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995245 Free PMC article.
-
Prophylactic alpha interferon treatment increases the therapeutic index of oncolytic vesicular stomatitis virus virotherapy for advanced hepatocellular carcinoma in immune-competent rats.J Virol. 2005 Nov;79(21):13705-13. doi: 10.1128/JVI.79.21.13705-13713.2005. J Virol. 2005. PMID: 16227290 Free PMC article.
-
Sensitivity of cervical carcinoma cells to vesicular stomatitis virus-induced oncolysis: potential role of human papilloma virus infection.Int J Cancer. 2012 Aug 1;131(3):E204-15. doi: 10.1002/ijc.27404. Epub 2012 Jan 11. Int J Cancer. 2012. PMID: 22173567
-
VSV-tumor selective replication and protein translation.Oncogene. 2005 Nov 21;24(52):7710-9. doi: 10.1038/sj.onc.1209042. Oncogene. 2005. PMID: 16299531 Review.
-
Vesicular stomatitis virus: an exciting new therapeutic oncolytic virus candidate for cancer or just another chapter from Field's Virology?Cancer Cell. 2003 Oct;4(4):241-3. doi: 10.1016/s1535-6108(03)00251-4. Cancer Cell. 2003. PMID: 14585348 Review.
Cited by
-
Oncolytic myxoma virus: the path to clinic.Vaccine. 2013 Sep 6;31(39):4252-8. doi: 10.1016/j.vaccine.2013.05.056. Epub 2013 May 29. Vaccine. 2013. PMID: 23726825 Free PMC article. Review.
-
Some attenuated variants of vesicular stomatitis virus show enhanced oncolytic activity against human glioblastoma cells relative to normal brain cells.J Virol. 2010 Feb;84(3):1563-73. doi: 10.1128/JVI.02040-09. Epub 2009 Nov 11. J Virol. 2010. PMID: 19906910 Free PMC article.
-
Inhibition of type I interferon-mediated antiviral action in human glioma cells by the IKK inhibitors BMS-345541 and TPCA-1.J Interferon Cytokine Res. 2012 Aug;32(8):368-77. doi: 10.1089/jir.2012.0002. Epub 2012 Apr 17. J Interferon Cytokine Res. 2012. PMID: 22509977 Free PMC article.
-
Extracellular matrix protein CCN1 limits oncolytic efficacy in glioma.Cancer Res. 2012 Mar 15;72(6):1353-62. doi: 10.1158/0008-5472.CAN-11-2526. Epub 2012 Jan 26. Cancer Res. 2012. PMID: 22282654 Free PMC article.
-
Attenuation of vesicular stomatitis virus infection of brain using antiviral drugs and an adeno-associated virus-interferon vector.Virology. 2015 Jan 15;475:1-14. doi: 10.1016/j.virol.2014.10.035. Epub 2014 Nov 21. Virology. 2015. PMID: 25462341 Free PMC article.
References
-
- Ahmed, M., S. D. Cramer, and D. S. Lyles. 2004. Sensitivity of prostate tumors to wild type and M protein mutant vesicular stomatitis viruses. Virology 330:34-49. - PubMed
-
- Barber, G. N. 2005. VSV-tumor selective replication and protein translation. Oncogene 24:7710-7719. - PubMed
-
- Chesler, D. A., and C. S. Reiss. 2002. The role of IFN-gamma in immune responses to viral infections of the central nervous system. Cytokine Growth Factor Rev. 13:441-454. - PubMed
-
- Chiocca, E. A. 2002. Oncolytic viruses. Nat. Rev. Cancer 2:938-950. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

