Characterization of protein kinase CK2 from Trypanosoma brucei
- PMID: 17097160
- PMCID: PMC1790856
- DOI: 10.1016/j.molbiopara.2006.10.002
Characterization of protein kinase CK2 from Trypanosoma brucei
Abstract
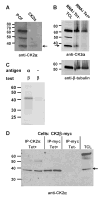
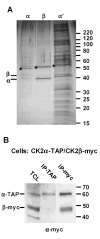
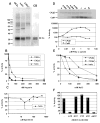
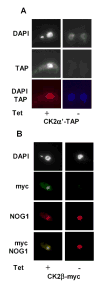
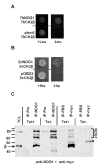
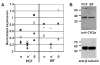
CK2 is a ubiquitous but enigmatic kinase. The difficulty in assigning a role to CK2 centers on the fact that, to date, no biologically relevant modulator of its function has been identified. One common theme revolves around a constellation of known substrates involved in growth control, compatible with its concentration in the nucleus and nucleolus. We had previously described the identification of two catalytic subunits of CK2 in Trypanosoma brucei and characterized one of them. Here we report the characterization of the second catalytic subunit, CK2alpha', and the identification and characterization of the regulatory subunit CK2beta. All three subunits are primarily localized to the nucleolus in T. brucei. We also show that CK2beta interacts with the nucleolar protein NOG1, adding to the interaction map which previously linked CK2alpha to the nucleolar protein NOPP44/46, which in turn associates with the rRNA binding protein p37. CK2 activity has four distinctive features: near equal affinity for GTP and ATP, heparin sensitivity, and stimulation by polyamines and polybasic peptides. Sequence comparison shows that the parasite orthologues have mutations in residues previously mapped as important in specifying affinity for GTP and stimulation by both polyamines and polybasic peptides. Studies of the enzymatic activity of the T. brucei CK2s show that both the affinity for GTP and stimulation by polyamines have been lost and only the features of heparin inhibition and stimulation by polybasic peptides are conserved.
Figures


Similar articles
-
Molecular cloning of Trypanosoma brucei CK2 catalytic subunits: the alpha isoform is nucleolar and phosphorylates the nucleolar protein Nopp44/46.Mol Biochem Parasitol. 2002 Jan;119(1):97-106. doi: 10.1016/s0166-6851(01)00407-8. Mol Biochem Parasitol. 2002. PMID: 11755190
-
Structure of the human protein kinase CK2 catalytic subunit CK2α' and interaction thermodynamics with the regulatory subunit CK2β.J Mol Biol. 2011 Mar 18;407(1):1-12. doi: 10.1016/j.jmb.2011.01.020. Epub 2011 Jan 15. J Mol Biol. 2011. PMID: 21241709
-
Toward selective CK2alpha and CK2alpha' inhibitors: Development of a novel whole-cell kinase assay by Autodisplay of catalytic CK2alpha'.J Pharm Biomed Anal. 2016 Mar 20;121:253-260. doi: 10.1016/j.jpba.2016.01.011. Epub 2016 Jan 8. J Pharm Biomed Anal. 2016. PMID: 26786382
-
Protein Kinase CK2α', More than a Backup of CK2α.Cells. 2023 Dec 14;12(24):2834. doi: 10.3390/cells12242834. Cells. 2023. PMID: 38132153 Free PMC article. Review.
-
Protein kinase CK2 in health and disease: Protein kinase CK2: from structures to insights.Cell Mol Life Sci. 2009 Jun;66(11-12):1800-16. doi: 10.1007/s00018-009-9149-8. Cell Mol Life Sci. 2009. PMID: 19387553 Free PMC article. Review.
Cited by
-
Functions and cellular localization of cysteine desulfurase and selenocysteine lyase in Trypanosoma brucei.FEBS J. 2010 Jan;277(2):383-93. doi: 10.1111/j.1742-4658.2009.07489.x. Epub 2009 Dec 7. FEBS J. 2010. PMID: 19968861 Free PMC article.
-
Structural and functional association of Trypanosoma brucei MIX protein with cytochrome c oxidase complex.Eukaryot Cell. 2008 Nov;7(11):1994-2003. doi: 10.1128/EC.00204-08. Epub 2008 Sep 5. Eukaryot Cell. 2008. PMID: 18776036 Free PMC article.
-
Functional characterization of two paralogs that are novel RNA binding proteins influencing mitochondrial transcripts of Trypanosoma brucei.RNA. 2012 Oct;18(10):1846-61. doi: 10.1261/rna.033852.112. Epub 2012 Aug 16. RNA. 2012. PMID: 22898985 Free PMC article.
-
The F(0)F(1)-ATP synthase complex contains novel subunits and is essential for procyclic Trypanosoma brucei.PLoS Pathog. 2009 May;5(5):e1000436. doi: 10.1371/journal.ppat.1000436. Epub 2009 May 15. PLoS Pathog. 2009. PMID: 19436713 Free PMC article.
-
Investigating RNA editing factors from trypanosome mitochondria.Methods. 2016 Sep 1;107:23-33. doi: 10.1016/j.ymeth.2016.03.020. Epub 2016 Mar 25. Methods. 2016. PMID: 27020893 Free PMC article.
References
-
- Dobrowolska G, Lozeman FJ, Li D, Krebs EG. CK2, a protein kinase of the next millennium. Mol Cell Biochem. 1999;191:3–12. - PubMed
-
- Glover CV. A filamentous form of Drosophila casein kinase II. J Biol Chem. 1986;261:14349–54. - PubMed
-
- Valero E, De BS, Filhol O, Wade RH, Langowski J, Chambaz EM, Cochet C. Quaternary structure of casein kinase 2. Characterization of multiple oligomeric states and relation with its catalytic activity. J Biol Chem. 1995;270:8345–52. - PubMed
-
- Abramczyk O, Zien P, Zielinski R, Pilecki M, Hellman U, Szyszka R. The protein kinase 60S is a free catalytic CK2alpha' subunit and forms an inactive complex with superoxide dismutase SOD1. Biochem Biophys Res Commun. 2003;307:31–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

