Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance
- PMID: 17088346
- PMCID: PMC1828419
- DOI: 10.1128/IAI.01475-06
Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance
Abstract
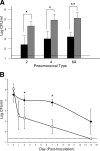
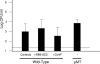
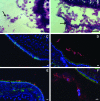
Expression of a polysaccharide capsule is required for the full pathogenicity of many mucosal pathogens such as Streptococcus pneumoniae. Although capsule allows for evasion of opsonization and subsequent phagocytosis during invasive infection, its role during mucosal colonization, the organism's commensal state, remains unknown. Using a mouse model, we demonstrate that unencapsulated mutants remain capable of nasal colonization but at a reduced density and duration compared to those of their encapsulated parent strains. This deficit in colonization was not due to increased susceptibility to opsonophagocytic clearance involving complement, antibody, or the influx of Ly-6G-positive cells, including neutrophils seen during carriage. Rather, unencapsulated mutants remain agglutinated within lumenal mucus and, thus, are less likely to transit to the epithelial surface where stable colonization occurs. Studies of in vitro binding to immobilized human airway mucus confirmed the inhibitory effect of encapsulation. Likewise, pneumococcal variants expressing larger amounts of negatively charged capsule per cell were less likely to adhere to surfaces coated with human mucus and more likely to evade initial clearance in vivo. Removal of negatively charged sialic acid residues by pretreatment of mucus with neuraminidase diminished the antiadhesive effect of encapsulation. This suggests that the inhibitory effect of encapsulation on mucus binding may be mediated by electrostatic repulsion and offers an explanation for the predominance of anionic polysaccharides among the diverse array of unique capsule types. In conclusion, our findings demonstrate that capsule confers an advantage to mucosal pathogens distinct from its role in inhibition of opsonophagocytosis--escape from entrapment in lumenal mucus.
Figures
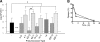

Similar articles
-
Capsule Type and Amount Affect Shedding and Transmission of Streptococcus pneumoniae.mBio. 2017 Aug 22;8(4):e00989-17. doi: 10.1128/mBio.00989-17. mBio. 2017. PMID: 28830943 Free PMC article.
-
Capsule Promotes Intracellular Survival and Vascular Endothelial Cell Translocation during Invasive Pneumococcal Disease.mBio. 2021 Oct 26;12(5):e0251621. doi: 10.1128/mBio.02516-21. Epub 2021 Oct 12. mBio. 2021. PMID: 34634940 Free PMC article.
-
The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease.Infect Immun. 2014 Feb;82(2):694-705. doi: 10.1128/IAI.01289-13. Epub 2013 Nov 25. Infect Immun. 2014. PMID: 24478084 Free PMC article.
-
Nonencapsulated Streptococcus pneumoniae: Emergence and Pathogenesis.mBio. 2016 Mar 22;7(2):e01792. doi: 10.1128/mBio.01792-15. mBio. 2016. PMID: 27006456 Free PMC article. Review.
-
Pneumococcal virulence factors and host immune responses to them.Eur J Clin Microbiol Infect Dis. 1995 Jun;14(6):479-90. doi: 10.1007/BF02113425. Eur J Clin Microbiol Infect Dis. 1995. PMID: 7588820 Review.
Cited by
-
Clinical implications of pneumococcal serotypes: invasive disease potential, clinical presentations, and antibiotic resistance.J Korean Med Sci. 2013 Jan;28(1):4-15. doi: 10.3346/jkms.2013.28.1.4. Epub 2013 Jan 8. J Korean Med Sci. 2013. PMID: 23341706 Free PMC article. Review.
-
Genetic and biochemical characterizations of enzymes involved in Streptococcus pneumoniae serotype 2 capsule synthesis demonstrate that Cps2T (WchF) catalyzes the committed step by addition of β1-4 rhamnose, the second sugar residue in the repeat unit.J Bacteriol. 2012 Dec;194(23):6479-89. doi: 10.1128/JB.01135-12. Epub 2012 Sep 21. J Bacteriol. 2012. PMID: 23002227 Free PMC article.
-
Pneumococci: immunology of the innate host response.Respirology. 2010 Oct;15(7):1057-63. doi: 10.1111/j.1440-1843.2010.01814.x. Epub 2010 Jul 20. Respirology. 2010. PMID: 20646240 Free PMC article. Review.
-
Characterization of inflammatory responses during intranasal colonization with Streptococcus pneumoniae.J Vis Exp. 2014 Jan 17;(83):e50490. doi: 10.3791/50490. J Vis Exp. 2014. PMID: 24472828 Free PMC article.
-
Biochemical activities of Streptococcus pneumoniae serotype 2 capsular glycosyltransferases and significance of suppressor mutations affecting the initiating glycosyltransferase Cps2E.J Bacteriol. 2013 Dec;195(24):5469-78. doi: 10.1128/JB.00715-13. Epub 2013 Oct 4. J Bacteriol. 2013. PMID: 24097952 Free PMC article.
References
-
- Bogaert, D., R. de Groot, and P. Hermans. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144-154. - PubMed
-
- Brown, E., S. Hosea, and M. Frank. 1983. The role of antibody and complement in the reticuloendothelial clearance of pneumococci from the bloodstream. Rev. Infect. Dis. 4:S797-S805. - PubMed
-
- Bryder, D., Y. Sasaki, O. Borge, and S.-E. Jacobsen. 2004. Deceptive multilineage reconstitution analysis of mice transplanted with hemopoietic stem cells, and implications for assessment of stem cell numbers and lineage potentials. J. Immunol. 172:1548-1552. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

