S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage
- PMID: 17078026
- PMCID: PMC2739421
- DOI: 10.1002/glia.20445
S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage
Abstract
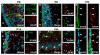
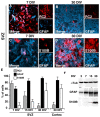
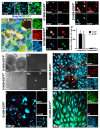
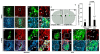
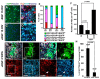
During the postnatal development, astrocytic cells in the neocortex progressively lose their neural stem cell (NSC) potential, whereas this peculiar attribute is preserved in the adult subventricular zone (SVZ). To understand this fundamental difference, many reports suggest that adult subventricular GFAP-expressing cells might be maintained in immature developmental stage. Here, we show that S100B, a marker of glial cells, is absent from GFAP-expressing cells of the SVZ and that its onset of expression characterizes a terminal maturation stage of cortical astrocytic cells. Nevertheless, when cultured in vitro, SVZ astrocytic cells developed as S100B expressing cells, as do cortical astrocytic cells, suggesting that SVZ microenvironment represses S100B expression. Using transgenic s100b-EGFP cells, we then demonstrated that S100B expression coincides with the loss of neurosphere forming abilities of GFAP expressing cells. By doing grafting experiments with cells derived from beta-actin-GFP mice, we next found that S100B expression in astrocytic cells is repressed in the SVZ, but not in the striatal parenchyma. Furthermore, we showed that treatment with epidermal growth factor represses S100B expression in GFAP-expressing cells in vitro as well as in vivo. Altogether, our results indicate that the S100B expression defines a late developmental stage after which GFAP-expressing cells lose their NSC potential and suggest that S100B expression is repressed by adult SVZ microenvironment.
Figures

Similar articles
-
Spatial and temporal expression of S100B in cells of oligodendrocyte lineage.Glia. 2005 Aug 1;51(2):81-97. doi: 10.1002/glia.20184. Glia. 2005. PMID: 15782413
-
Glial fibrillary acidic protein-expressing neural progenitors give rise to immature neurons via early intermediate progenitors expressing both glial fibrillary acidic protein and neuronal markers in the adult hippocampus.Neuroscience. 2010 Mar 10;166(1):241-51. doi: 10.1016/j.neuroscience.2009.12.026. Epub 2009 Dec 16. Neuroscience. 2010. PMID: 20026190
-
GFAP-expressing cells in the postnatal subventricular zone display a unique glial phenotype intermediate between radial glia and astrocytes.Glia. 2006 Oct;54(5):394-410. doi: 10.1002/glia.20392. Glia. 2006. PMID: 16886203
-
Identity crisis for adult periventricular neural stem cells: subventricular zone astrocytes, ependymal cells or both?Nat Rev Neurosci. 2009 Feb;10(2):153-63. doi: 10.1038/nrn2571. Nat Rev Neurosci. 2009. PMID: 19153578 Review.
-
S100B in brain damage and neurodegeneration.Microsc Res Tech. 2003 Apr 15;60(6):614-32. doi: 10.1002/jemt.10303. Microsc Res Tech. 2003. PMID: 12645009 Review.
Cited by
-
Serum S100B represents a new biomarker for mood disorders.Curr Drug Targets. 2013 Oct;14(11):1237-48. doi: 10.2174/13894501113149990014. Curr Drug Targets. 2013. PMID: 23701298 Free PMC article. Review.
-
Isolation of endothelial cells, pericytes and astrocytes from mouse brain.PLoS One. 2019 Dec 18;14(12):e0226302. doi: 10.1371/journal.pone.0226302. eCollection 2019. PLoS One. 2019. PMID: 31851695 Free PMC article.
-
Simultaneous brain cell type and lineage determined by scRNA-seq reveals stereotyped cortical development.Cell Syst. 2022 Jun 15;13(6):438-453.e5. doi: 10.1016/j.cels.2022.03.006. Epub 2022 Apr 21. Cell Syst. 2022. PMID: 35452605 Free PMC article.
-
Excitatory Amino Acid Transporters in Physiology and Disorders of the Central Nervous System.Int J Mol Sci. 2019 Nov 12;20(22):5671. doi: 10.3390/ijms20225671. Int J Mol Sci. 2019. PMID: 31726793 Free PMC article. Review.
-
Upregulation of Neural Cell Adhesion Molecule 1 and Excessive Migration of Purkinje Cells in Cerebellar Cortex.Front Neurosci. 2022 Jan 21;15:804402. doi: 10.3389/fnins.2021.804402. eCollection 2021. Front Neurosci. 2022. PMID: 35126044 Free PMC article.
References
-
- Ahn S, Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. - PubMed
-
- Anthony TE, Klein C, Fishell G, Heintz N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron. 2004;41:881–90. - PubMed
-
- Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–14. - PubMed
-
- Burrows RC, Wancio D, Levitt P, Lillien L. Response diversity and the timing of progenitor cell maturation are regulated by developmental changes in EGFR expression in the cortex. Neuron. 1997;19:251–67. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

