The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis
- PMID: 17070835
- PMCID: PMC2577175
- DOI: 10.1016/j.jinsphys.2006.09.004
The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis
Abstract
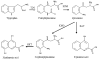
Oxidation of tryptophan to kynurenine and 3-hydroxykynurenine (3-HK) is the major catabolic pathway in mosquitoes. However, 3-HK is oxidized easily under physiological conditions, resulting in the production of reactive radical species. To overcome this problem, mosquitoes have developed an efficient mechanism to prevent 3-HK from accumulating by converting this chemically reactive compound to the chemically stable xanthurenic acid. Interestingly, 3-HK is a precursor for the production of compound eye pigments during the pupal and early adult stages; consequently, mosquitoes need to preserve and transport 3-HK for compound eye pigmentation in pupae and adults. This review summarizes the tryptophan oxidation pathway, compares and contrasts the mosquito tryptophan oxidation pathway with other model species, and discusses possible driving forces leading to the functional adaptation and evolution of enzymes involved in the mosquito tryptophan oxidation pathway.
Figures


Similar articles
-
Oxidation of 3-hydroxykynurenine to produce xanthommatin for eye pigmentation: a major branch pathway of tryptophan catabolism during pupal development in the yellow fever mosquito, Aedes aegypti.Insect Biochem Mol Biol. 1999 Apr;29(4):329-38. doi: 10.1016/s0965-1748(99)00007-7. Insect Biochem Mol Biol. 1999. PMID: 10333572
-
Identification and biochemical characterization of the Anopheles gambiae 3-hydroxykynurenine transaminase.FEBS J. 2005 Nov;272(21):5653-62. doi: 10.1111/j.1742-4658.2005.04961.x. FEBS J. 2005. PMID: 16262702
-
Transamination of 3-hydroxykynurenine to produce xanthurenic acid: a major branch pathway of tryptophan metabolism in the mosquito, Aedes aegypti, during larval development.Insect Biochem Mol Biol. 1997 Oct;27(10):859-67. doi: 10.1016/s0965-1748(97)00068-4. Insect Biochem Mol Biol. 1997. PMID: 9474782
-
[Enzymes of the kynurenine pathway].Postepy Hig Med Dosw. 2001;55(5):715-31. Postepy Hig Med Dosw. 2001. PMID: 11795205 Review. Polish.
-
[Kynurenines in pathogenesis of endogenous psychiatric disorders].Vestn Ross Akad Med Nauk. 2013;(1):35-41. doi: 10.15690/vramn.v68i1.535. Vestn Ross Akad Med Nauk. 2013. PMID: 23805637 Review. Russian.
Cited by
-
Developmental and Nutritional Dynamics of Malpighian Tubule Autofluorescence in the Asian Tiger Mosquito Aedes albopictus.Int J Mol Sci. 2023 Dec 23;25(1):245. doi: 10.3390/ijms25010245. Int J Mol Sci. 2023. PMID: 38203417 Free PMC article.
-
The antioxidant role of xanthurenic acid in the Aedes aegypti midgut during digestion of a blood meal.PLoS One. 2012;7(6):e38349. doi: 10.1371/journal.pone.0038349. Epub 2012 Jun 11. PLoS One. 2012. PMID: 22701629 Free PMC article.
-
Insecticidal activity of indole derivatives against Plutella xylostella and selectivity to four non-target organisms.Ecotoxicology. 2019 Oct;28(8):973-982. doi: 10.1007/s10646-019-02095-1. Epub 2019 Aug 16. Ecotoxicology. 2019. PMID: 31420785
-
Effect of marker-free transgenic Chlamydomonas on the control of Aedes mosquito population and on plankton.Parasit Vectors. 2023 Jan 18;16(1):18. doi: 10.1186/s13071-022-05647-3. Parasit Vectors. 2023. PMID: 36653886 Free PMC article.
-
Next-generation gene drive for population modification of the malaria vector mosquito, Anopheles gambiae.Proc Natl Acad Sci U S A. 2020 Sep 15;117(37):22805-22814. doi: 10.1073/pnas.2010214117. Epub 2020 Aug 24. Proc Natl Acad Sci U S A. 2020. PMID: 32839345 Free PMC article.
References
-
- Akaboshi E. Kynurenine hydroxylase in Musca domestica L. Comparative Biochemistry and Physiology B. 1979;62:549–555. - PubMed
-
- Arndt R, Junge W, Michelssen K, Krisch K. Isolation and molecular properties of formamidase from rat liver cytoplasm. HoppeSeyler’s Zeitschrift für Physiologische Chemie. 1973;354:1583–1590. - PubMed
-
- Atkinson PW, Pinkerton AC, O’Brochta DA. Genetic transformation systems in insects. Annual Review of Entomology. 2001;46:317–346. - PubMed
-
- Bailey CG, Wagner C. Kynurenine formamidase. Purification and characterization of the adult chicken liver enzyme and immuno-chemical analyses of the enzyme of developing chicks. Journal of Biological Chemistry. 1974;249:4439–4444. - PubMed
-
- Baillie DL, Chovnick A. Studies on the genetic control of tryptophan pyrrolase in Drosophila melanogaster. Molecular and General Genetics. 1971;112:341–353. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

