Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways
- PMID: 17064973
- PMCID: PMC1913208
- DOI: 10.1016/j.bone.2006.09.011
Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways
Abstract
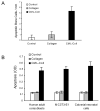
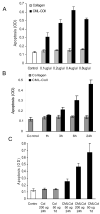
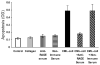
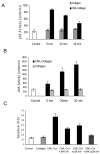
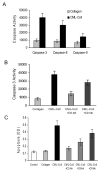
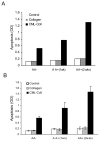
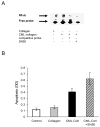
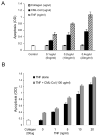
We have previously shown that diabetes significantly enhances apoptosis of osteoblastic cells in vivo and that the enhanced apoptosis contributes to diabetes impaired new bone formation. A potential mechanism is enhanced apoptosis stimulated by advanced glycation end products (AGEs). To investigate this further, an advanced glycation product, carboxymethyl lysine modified collagen (CML-collagen), was injected in vivo and stimulated a 5-fold increase in calvarial periosteal cell apoptosis compared to unmodified collagen. It also induced apoptosis in primary cultures of human or neonatal rat osteoblastic cells or MC3T3-E1 cells in vitro. Moreover, the apoptotic effect was largely mediated through RAGE receptor. CML-collagen increased p38 and JNK activity 3.2- and 4.4-fold, respectively. Inhibition of p38 and JNK reduced CML-collagen stimulated apoptosis by 45% and 59% and by 90% when used together (P<0.05). The predominant apoptotic pathway induced by CML-collagen involved caspase-8 activation of caspase-3 and was independent of NF-kappaB activation. When osteoblastic cells were exposed to a long-term low dose incubation with CML-collagen, there was a higher degree of apoptosis compared to short-term incubation. In more differentiated osteoblastic cultures, apoptosis was enhanced even further. These results indicate that advanced glycation end products, which accumulate in diabetic and aged individuals, may promote apoptosis of osteoblastic cells and contribute to deficient bone formation.
Figures
Similar articles
-
Advanced glycation end products induce apoptosis in fibroblasts through activation of ROS, MAP kinases, and the FOXO1 transcription factor.Am J Physiol Cell Physiol. 2007 Feb;292(2):C850-6. doi: 10.1152/ajpcell.00356.2006. Epub 2006 Sep 27. Am J Physiol Cell Physiol. 2007. PMID: 17005604
-
Advanced glycation end products enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways.J Biol Chem. 2005 Apr 1;280(13):12087-95. doi: 10.1074/jbc.M406313200. Epub 2004 Dec 6. J Biol Chem. 2005. PMID: 15590648
-
Collagen advanced glycation inhibits its Discoidin Domain Receptor 2 (DDR2)-mediated induction of lysyl oxidase in osteoblasts.Bone. 2014 Jan;58:33-41. doi: 10.1016/j.bone.2013.10.001. Epub 2013 Oct 10. Bone. 2014. PMID: 24120383 Free PMC article.
-
Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation.Glycobiology. 2005 Jul;15(7):16R-28R. doi: 10.1093/glycob/cwi053. Epub 2005 Mar 10. Glycobiology. 2005. PMID: 15764591 Review.
-
The role of AGEs in aging: causation or correlation.Exp Gerontol. 2001 Sep;36(9):1527-37. doi: 10.1016/s0531-5565(01)00138-3. Exp Gerontol. 2001. PMID: 11525875 Review.
Cited by
-
Dietary marine hydrolysate alleviates D-galactose-induced brain aging by attenuating cognitive alterations, oxidative stress and inflammation through the AGE-RAGE axis.PLoS One. 2024 Oct 24;19(10):e0309542. doi: 10.1371/journal.pone.0309542. eCollection 2024. PLoS One. 2024. PMID: 39446794 Free PMC article.
-
The complex relationship between bone remodeling and the physical and material properties of bone.Osteoporos Int. 2015 Mar;26(3):845-7. doi: 10.1007/s00198-014-2970-4. Epub 2014 Dec 20. Osteoporos Int. 2015. PMID: 25526711 No abstract available.
-
Diabetes Stimulates Osteoclastogenesis by Acidosis-Induced Activation of Transient Receptor Potential Cation Channels.Sci Rep. 2016 Jul 29;6:30639. doi: 10.1038/srep30639. Sci Rep. 2016. PMID: 27468810 Free PMC article.
-
Advanced glycation end products induce human corneal epithelial cells apoptosis through generation of reactive oxygen species and activation of JNK and p38 MAPK pathways.PLoS One. 2013 Jun 12;8(6):e66781. doi: 10.1371/journal.pone.0066781. Print 2013. PLoS One. 2013. PMID: 23776698 Free PMC article.
-
Advanced glycation end products promote osteoporosis by inducing ferroptosis in osteoblasts.Mol Med Rep. 2022 Apr;25(4):140. doi: 10.3892/mmr.2022.12656. Epub 2022 Feb 25. Mol Med Rep. 2022. PMID: 35211757 Free PMC article.
References
-
- Akin O, Gol K, Akturk M, Erkaya S. Evaluation of bone turnover in postmenopausal patients with type 2 diabetes mellitus using biochemical markers and bone mineral density measurements. Gynecol Endocrinol. 2003;17:19–29. - PubMed
-
- Alikhani M, Alikhani Z, Graves D. FOXO1 functions as a master switch that regulates gene expression necessary for TNF-induced fibroblast apoptosis. J Biol Chem. 2005;280:12096–102. - PubMed
-
- Alikhani M, Alikhani Z, Raptis M, Graves D. TNF-alpha in vivo stimulates apoptosis in fibroblasts through caspase-8 activation and modulates the expression of pro-apoptotic genes. J Cell Physiol. 2005;201:341–8. - PubMed
-
- Alikhani Z, Alikhani M, Boyd C, Nagao K, Trackman P, Graves D. Advanced glycation endproducts enhance expression of pro-apoptotic genes and stimulate fibroblast apoptosis through cytoplasmic and mitochondrial pathways. J Biol Chem. 2005;280:12087–95. - PubMed
-
- Al-Mashat HA, Kandru S, Liu R, Behl Y, Desta T, Graves DT. Diabetes enhances mRNA levels of proapoptotic genes and caspase activity, which contribute to impaired healing. Diabetes. 2006;55:487–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

