Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS
- PMID: 17055560
- PMCID: PMC1778460
- DOI: 10.1016/j.yfrne.2006.09.004
Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS
Abstract
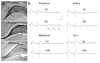
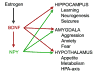
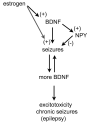
In the CNS, there are widespread and diverse interactions between growth factors and estrogen. Here we examine the interactions of estrogen and brain-derived neurotrophic factor (BDNF), two molecules that have historically been studied separately, despite the fact that they seem to share common targets, effects, and mechanisms of action. The demonstration of an estrogen-sensitive response element on the BDNF gene provided an impetus to explore a direct relationship between estrogen and BDNF, and predicted that the effects of estrogen, at least in part, might be due to the induction of BDNF. This hypothesis is discussed with respect to the hippocampus, where substantial evidence has accumulated in favor of it, but alternate hypotheses are also raised. It is suggested that some of the interactions between estrogen and BDNF, as well as the controversies and implications associated with their respective actions, may be best appreciated in light of the ability of BDNF to induce neuropeptide Y (NPY) synthesis in hippocampal neurons. Taken together, this tri-molecular cascade, estrogen-BDNF-NPY, may be important in understanding the hormonal regulation of hippocampal function. It may also be relevant to other regions of the CNS where estrogen is known to exert profound effects, such as amygdala and hypothalamus; and may provide greater insight into neurological disorders and psychiatric illness, including Alzheimer's disease, depression and epilepsy.
Figures
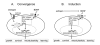

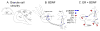

Similar articles
-
Similarities between actions of estrogen and BDNF in the hippocampus: coincidence or clue?Trends Neurosci. 2005 Feb;28(2):79-85. doi: 10.1016/j.tins.2004.12.005. Trends Neurosci. 2005. PMID: 15667930 Review.
-
Estrogen-growth factor interactions and their contributions to neurological disorders.Headache. 2008 Jul;48 Suppl 2(Suppl 2):S77-89. doi: 10.1111/j.1526-4610.2008.01200.x. Headache. 2008. PMID: 18700946 Free PMC article. Review.
-
Endogenous control of hippocampal epileptogenesis: a molecular cascade involving brain-derived neurotrophic factor and neuropeptide Y.Epilepsia. 2000;41 Suppl 6:S127-33. doi: 10.1111/j.1528-1157.2000.tb01571.x. Epilepsia. 2000. PMID: 10999534
-
Brain-derived neurotrophic factor-estrogen interactions in the hippocampal mossy fiber pathway: implications for normal brain function and disease.Neuroscience. 2013 Jun 3;239:46-66. doi: 10.1016/j.neuroscience.2012.12.029. Epub 2012 Dec 29. Neuroscience. 2013. PMID: 23276673 Free PMC article. Review.
-
Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus.Exp Neurol. 2005 Oct;195(2):353-71. doi: 10.1016/j.expneurol.2005.05.014. Exp Neurol. 2005. PMID: 16002067
Cited by
-
Socially modulated cell proliferation is independent of gonadal steroid hormones in the brain of the adult green treefrog (Hyla cinerea).Brain Behav Evol. 2012;79(3):170-80. doi: 10.1159/000335037. Epub 2012 Jan 20. Brain Behav Evol. 2012. PMID: 22269468 Free PMC article.
-
Testosterone and brain-derived neurotrophic factor interactions in the avian song control system.Neuroscience. 2013 Jun 3;239:115-23. doi: 10.1016/j.neuroscience.2012.09.023. Epub 2012 Oct 30. Neuroscience. 2013. PMID: 23123886 Free PMC article. Review.
-
Central depletion of brain-derived neurotrophic factor in mice results in high bone mass and metabolic phenotype.Endocrinology. 2012 Nov;153(11):5394-405. doi: 10.1210/en.2012-1378. Epub 2012 Sep 25. Endocrinology. 2012. PMID: 23011922 Free PMC article.
-
Effects of voluntary running in the female mice lateral septum on BDNF and corticotropin-releasing factor receptor 2.Int J Pept. 2011;2011:932361. doi: 10.1155/2011/932361. Epub 2011 Sep 29. Int J Pept. 2011. PMID: 21977046 Free PMC article.
-
Reiterated male-to-female violence disrupts hippocampal estrogen receptor β expression, prompting anxiety-like behavior.iScience. 2024 Jul 25;27(9):110585. doi: 10.1016/j.isci.2024.110585. eCollection 2024 Sep 20. iScience. 2024. PMID: 39228787 Free PMC article.
References
-
- Stancel GM, Gardner RM, Kirkland JL, Lin TH, Lingham RB, Loose-Mitchell DS, Mukku VR, Orengo CA, Verner G. Interactions between estrogen and EGF in uterine growth and function. Adv Exp Med Biol. 1987;230:99–118. - PubMed
-
- Mendez P, Cardona-Gomez GP, Garcia-Segura LM. Interactions of insulin-like growth factor-I and estrogen in the brain. Adv Exp Med Biol. 2005;567:285–303. - PubMed
-
- Toran-Allerand CD, Singh M, Setalo G. Novel mechanisms of estrogen action in the brain: new players in an old story. Front Neuroendocrinol. 1999;20:97–121. - PubMed
-
- Sandstrom NJ, Williams CL. Spatial memory retention is enhanced by acute and continuous estradiol replacement. Horm Behav. 2004;45:128–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

