A genetic variant that disrupts MET transcription is associated with autism
- PMID: 17053076
- PMCID: PMC1838551
- DOI: 10.1073/pnas.0605296103
A genetic variant that disrupts MET transcription is associated with autism
Abstract
There is strong evidence for a genetic predisposition to autism and an intense interest in discovering heritable risk factors that disrupt gene function. Based on neurobiological findings and location within a chromosome 7q31 autism candidate gene region, we analyzed the gene encoding the pleiotropic MET receptor tyrosine kinase in a family based study of autism including 1,231 cases. MET signaling participates in neocortical and cerebellar growth and maturation, immune function, and gastrointestinal repair, consistent with reported medical complications in some children with autism. Here, we show genetic association (P = 0.0005) of a common C allele in the promoter region of the MET gene in 204 autism families. The allelic association at this MET variant was confirmed in a replication sample of 539 autism families (P = 0.001) and in the combined sample (P = 0.000005). Multiplex families, in which more than one child has autism, exhibited the strongest allelic association (P = 0.000007). In case-control analyses, the autism diagnosis relative risk was 2.27 (95% confidence interval: 1.41-3.65; P = 0.0006) for the CC genotype and 1.67 (95% confidence interval: 1.11-2.49; P = 0.012) for the CG genotype compared with the GG genotype. Functional assays showed that the C allele results in a 2-fold decrease in MET promoter activity and altered binding of specific transcription factor complexes. These data implicate reduced MET gene expression in autism susceptibility, providing evidence of a previously undescribed pathophysiological basis for this behaviorally and medically complex disorder.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

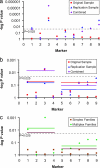
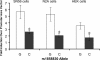

Comment in
-
A surprising METamorphosis: autism genetics finds a common functional variant.Proc Natl Acad Sci U S A. 2006 Nov 7;103(45):16621-2. doi: 10.1073/pnas.0608027103. Epub 2006 Oct 30. Proc Natl Acad Sci U S A. 2006. PMID: 17075042 Free PMC article. No abstract available.
Similar articles
-
Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions.Pediatrics. 2009 Mar;123(3):1018-24. doi: 10.1542/peds.2008-0819. Pediatrics. 2009. PMID: 19255034
-
Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder.Autism Res. 2009 Aug;2(4):232-6. doi: 10.1002/aur.87. Autism Res. 2009. PMID: 19681062
-
Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder.Autism Res. 2008 Jun;1(3):159-68. doi: 10.1002/aur.27. Autism Res. 2008. PMID: 19360663 Free PMC article.
-
The genetics of autism.Pediatrics. 2004 May;113(5):e472-86. doi: 10.1542/peds.113.5.e472. Pediatrics. 2004. PMID: 15121991 Review.
-
The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders.J Clin Invest. 2009 Apr;119(4):747-54. doi: 10.1172/JCI37934. Epub 2009 Apr 1. J Clin Invest. 2009. PMID: 19339766 Free PMC article. Review.
Cited by
-
Early-Life Stress Paradigm Transiently Alters Maternal Behavior, Dam-Pup Interactions, and Offspring Vocalizations in Mice.Front Behav Neurosci. 2016 Jul 5;10:142. doi: 10.3389/fnbeh.2016.00142. eCollection 2016. Front Behav Neurosci. 2016. PMID: 27458353 Free PMC article.
-
Synaptic and extrasynaptic location of the receptor tyrosine kinase met during postnatal development in the mouse neocortex and hippocampus.J Comp Neurol. 2013 Oct 1;521(14):3241-59. doi: 10.1002/cne.23343. J Comp Neurol. 2013. PMID: 23787772 Free PMC article.
-
High-resolution chromosome ideogram representation of currently recognized genes for autism spectrum disorders.Int J Mol Sci. 2015 Mar 20;16(3):6464-95. doi: 10.3390/ijms16036464. Int J Mol Sci. 2015. PMID: 25803107 Free PMC article.
-
Discovery of a novel cytokine signature for the diagnosis of autism spectrum disorder in young Arab children in Qatar.Front Psychiatry. 2024 Feb 13;15:1333534. doi: 10.3389/fpsyt.2024.1333534. eCollection 2024. Front Psychiatry. 2024. PMID: 38414501 Free PMC article.
-
Early medical and behavioral characteristics of NICU infants later classified with ASD.Pediatrics. 2010 Sep;126(3):457-67. doi: 10.1542/peds.2009-2680. Epub 2010 Aug 2. Pediatrics. 2010. PMID: 20679296 Free PMC article.
References
-
- Muhle R, Trentacoste SV, Rapin I. Pediatrics. 2004;113:e472–e486. - PubMed
-
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. J Am Med Assoc. 2003;289:49–55. - PubMed
-
- Le Couteur A, Bailey A, Goode S, Pickles A, Robertson S, Gottesman I, Rutter M. J Child Psychol Psychiatry. 1996;37:785–801. - PubMed
-
- Fombonne E. J Autism Dev Disord. 2003;33:365–382. - PubMed
-
- Barrett S, Beck JC, Bernier R, Bisson E, Braun TA, Casavant TL, Childress D, Folstein SE, Garcia M, Gardiner MB, et al. Am J Med Genet. 1999;88:609–615. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

