Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells
- PMID: 17050597
- PMCID: PMC1797244
- DOI: 10.1128/JVI.01303-06
Cell-free human immunodeficiency virus type 1 transcytosis through primary genital epithelial cells
Abstract
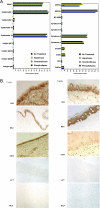
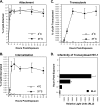
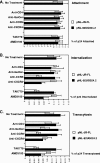
Although the transport of human immunodeficiency virus type 1 (HIV-1) through the epithelium is critical for HIV-1 colonization, the mechanisms controlling this process remain obscure. In the present study, we investigated the transcellular migration of HIV-1 as a cell-free virus through primary genital epithelial cells (PGECs). The absence of CD4 on PGECs implicates an unusual entry pathway for HIV-1. We found that syndecans are abundantly expressed on PGECs and promote the initial attachment and subsequent entry of HIV-1 through PGECs. Although CXCR4 and CCR5 do not contribute to HIV-1 attachment, they enhance viral entry and transcytosis through PGECs. Importantly, HIV-1 exploits both syndecans and chemokine receptors to ensure successful cell-free transport through the genital epithelium. HIV-1-syndecan interactions rely on specific residues in the V3 of gp120 and specific sulfations within syndecans. We found no obvious correlation between coreceptor usage and the capacity of the virus to transcytose. Since viruses isolated after sexual transmission are mainly R5 viruses, this suggests that the properties conferring virus replication after transmission are distinct from those conferring cell-free virus transcytosis through the genital epithelium. Although we found that cell-free HIV-1 crosses PGECs as infectious particles, the efficiency of transcytosis is extremely poor (less than 0.02% of the initial inoculum). This demonstrates that the genital epithelium serves as a major barrier against HIV-1. Although one cannot exclude the possibility that limited passage of cell-free HIV-1 transcytosis through an intact genital epithelium occurs in vivo, it is likely that the establishment of infection via cell-free HIV-1 transmigration is a rare event.
Figures



Similar articles
-
R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission.Virology. 2007 Feb 5;358(1):55-68. doi: 10.1016/j.virol.2006.07.029. Epub 2006 Aug 24. Virology. 2007. PMID: 16934308
-
Cryptic nature of a conserved, CD4-inducible V3 loop neutralization epitope in the native envelope glycoprotein oligomer of CCR5-restricted, but not CXCR4-using, primary human immunodeficiency virus type 1 strains.J Virol. 2005 Jun;79(11):6957-68. doi: 10.1128/JVI.79.11.6957-6968.2005. J Virol. 2005. PMID: 15890935 Free PMC article.
-
Interactions of CCR5 and CXCR4 with CD4 and gp120 in human blood monocyte-derived dendritic cells.Exp Mol Pathol. 2000 Jun;68(3):133-8. doi: 10.1006/exmp.1999.2300. Exp Mol Pathol. 2000. PMID: 10816381
-
Effect of HIV-1 subtype and tropism on treatment with chemokine coreceptor entry inhibitors; overview of viral entry inhibition.Crit Rev Microbiol. 2015;41(4):473-87. doi: 10.3109/1040841X.2013.867829. Epub 2014 Mar 17. Crit Rev Microbiol. 2015. PMID: 24635642 Review.
-
Human immunodeficiency virus type 1 entry and chemokine receptors: a new therapeutic target.Antivir Chem Chemother. 1999 Mar;10(2):53-62. doi: 10.1177/095632029901000201. Antivir Chem Chemother. 1999. PMID: 10335399 Review.
Cited by
-
Sublimable C5A delivery provides sustained and prolonged anti-HIV microbicidal activities.Antimicrob Agents Chemother. 2012 Jun;56(6):3336-43. doi: 10.1128/AAC.00186-12. Epub 2012 Mar 19. Antimicrob Agents Chemother. 2012. PMID: 22430971 Free PMC article.
-
Anti-HIV-1 activity of elafin is more potent than its precursor's, trappin-2, in genital epithelial cells.J Virol. 2012 Apr;86(8):4599-610. doi: 10.1128/JVI.06561-11. Epub 2012 Feb 15. J Virol. 2012. PMID: 22345469 Free PMC article.
-
The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways.Blood. 2008 Aug 15;112(4):1299-307. doi: 10.1182/blood-2008-01-136473. Epub 2008 Jun 9. Blood. 2008. PMID: 18541725 Free PMC article.
-
Multiple receptor interactions trigger release of membrane and intracellular calcium stores critical for herpes simplex virus entry.Mol Biol Cell. 2007 Aug;18(8):3119-30. doi: 10.1091/mbc.e07-01-0062. Epub 2007 Jun 6. Mol Biol Cell. 2007. PMID: 17553929 Free PMC article.
-
HIV-1 transmission: modelling and direct visualization in the third dimension.Microscopy (Oxf). 2023 Jun 8;72(3):164-177. doi: 10.1093/jmicro/dfad014. Microscopy (Oxf). 2023. PMID: 36762762 Free PMC article. Review.
References
-
- Annan, K. A. 2002. In Africa, AIDS has a woman's face. New York Times 29 December p. 9.
-
- Argyris, E. G., E. Acheampong, G. Nunnari, M. Mukhtar, J. K. Williams, and R. J. Pomerantz. 2003. Human immunodeficiency virus type 1 enters primary human brain microvascular endothelial cells by a mechanism involving cell surface proteoglycans independent of lipid rafts. J. Virol. 77:12140-12151. - PMC - PubMed
-
- Auvert, B., A. Buve, B. Ferry, M. Carael, L. Morison, E. Lagarde, N. J. Robinson, M. Kahindo, J. Chege, N. Rutenberg, R. Musonda, M. Laourou, E. Akam, and the Study Group on the Heterogeneity of HIV Epidemics in African Cities. 2001. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS 15(Suppl. 4):S15-S30. - PubMed
-
- Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

