Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines
- PMID: 17050593
- PMCID: PMC1797237
- DOI: 10.1128/JVI.01957-06
Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines
Abstract
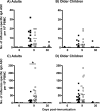
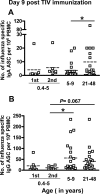
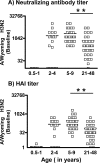
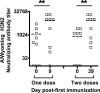
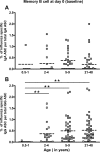
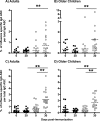
Cellular immune responses to influenza virus infection and influenza virus vaccination have not been rigorously characterized. We quantified the effector and memory B-cell responses in children and adults after administration of either live attenuated (LAIV) or inactivated (TIV) influenza virus vaccines and compared these to antibody responses. Peripheral blood mononuclear cells were collected at days 0, 7 to 12, and 27 to 42 after immunization of younger children (6 months to 4 years old), older children (5 to 9 years old), and adults. Influenza virus-specific effector immunoglobulin A (IgA) and IgG circulating antibody-secreting cells (ASC) and stimulated memory B cells were detected using an enzyme-linked immunospot assay. Circulating influenza virus-specific IgG and IgA ASC were detected 7 to 12 days after TIV and after LAIV immunization. Seventy-nine percent or more of adults and older children had demonstrable IgG ASC responses, while IgA ASC responses were detected in 29 to 53% of the subjects. The IgG ASC response rate to LAIV immunization in adults was significantly higher than the response rate measured by standard serum antibody assays (26.3% and 15.8% by neutralization and hemagglutination inhibition assays, respectively). IgG ASC and serum antibody responses were relatively low in the younger children compared to older children and adults. TIV, but not LAIV, significantly increased the percentage of circulating influenza virus-specific memory B cells detected at 27 to 42 days after immunization in children and adults. In conclusion, although both influenza vaccines are effective, we found significant differences in the B-cell and antibody responses elicited after LAIV or TIV immunization in adults and older children and between young children and older age groups.
Figures

Similar articles
-
Vaccine-specific antibody secreting cells are a robust early marker of LAIV-induced B-cell response in ferrets.Vaccine. 2012 Jan 5;30(2):237-46. doi: 10.1016/j.vaccine.2011.11.001. Epub 2011 Nov 12. Vaccine. 2012. PMID: 22080173
-
Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets.J Infect Dis. 2013 Aug 15;208(4):594-602. doi: 10.1093/infdis/jit207. Epub 2013 May 8. J Infect Dis. 2013. PMID: 23656978
-
Influence of prior influenza vaccination on antibody and B-cell responses.PLoS One. 2008 Aug 20;3(8):e2975. doi: 10.1371/journal.pone.0002975. PLoS One. 2008. PMID: 18714352 Free PMC article.
-
Seasonal influenza vaccines.Curr Top Microbiol Immunol. 2009;333:43-82. doi: 10.1007/978-3-540-92165-3_3. Curr Top Microbiol Immunol. 2009. PMID: 19768400 Review.
-
The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults.Influenza Other Respir Viruses. 2011 Mar;5(2):67-75. doi: 10.1111/j.1750-2659.2010.00183.x. Epub 2010 Nov 19. Influenza Other Respir Viruses. 2011. PMID: 21306569 Free PMC article. Review.
Cited by
-
Cell culture-based influenza vaccines: A necessary and indispensable investment for the future.Hum Vaccin Immunother. 2015;11(5):1223-34. doi: 10.1080/21645515.2015.1016666. Hum Vaccin Immunother. 2015. PMID: 25875691 Free PMC article. Review.
-
Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies.J Clin Invest. 2011 Aug;121(8):3109-19. doi: 10.1172/JCI57834. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785218 Free PMC article.
-
Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels.Proc Natl Acad Sci U S A. 2009 Mar 10;106(10):3877-82. doi: 10.1073/pnas.0813390106. Epub 2009 Feb 23. Proc Natl Acad Sci U S A. 2009. PMID: 19237568 Free PMC article. Clinical Trial.
-
Live attenuated influenza vaccine (FluMist®; Fluenz™): a review of its use in the prevention of seasonal influenza in children and adults.Drugs. 2011 Aug 20;71(12):1591-622. doi: 10.2165/11206860-000000000-00000. Drugs. 2011. PMID: 21861544 Review.
-
B Cells and Functional Antibody Responses to Combat Influenza.Front Immunol. 2015 Jun 30;6:336. doi: 10.3389/fimmu.2015.00336. eCollection 2015. Front Immunol. 2015. PMID: 26175732 Free PMC article. Review.
References
-
- Agresti, A. 1992. A survey of exact inference for contingency tables. Statistical Sci. 7:131-177.
-
- Belshe, R. B., and W. C. Gruber. 2000. Prevention of otitis media in children with live attenuated influenza vaccine given intranasally. Pediatr. Infect. Dis. J. 19:S66-S71. - PubMed
-
- Belshe, R. B., W. C. Gruber, P. M. Mendelman, H. B. Mehta, K. Mahmood, K. Reisinger, J. Treanor, K. Zangwill, F. G. Hayden, D. I. Bernstein, K. Kotloff, J. King, P. A. Piedra, S. L. Block, L. Yan, and M. Wolff. 2000. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J. Infect. Dis. 181:1133-1137. - PubMed
-
- Belshe, R. B., P. M. Mendelman, J. Treanor, J. King, W. C. Gruber, P. Piedra, D. I. Bernstein, F. G. Hayden, K. Kotloff, K. Zangwill, D. Iacuzio, and M. Wolff. 1998. The efficacy of live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine in children. N. Engl. J. Med. 338:1405-1412. - PubMed
-
- Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

