Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells
- PMID: 17041213
- PMCID: PMC1641800
- DOI: 10.1128/JVI.00939-06
Postentry events are responsible for restriction of productive varicella-zoster virus infection in Chinese hamster ovary cells
Abstract
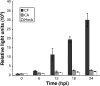
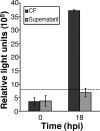
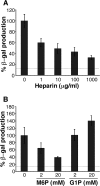
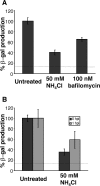
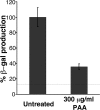
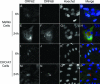
Productive infection of varicella-zoster virus (VZV) in vitro is restricted almost exclusively to cells derived from humans and other primates. We demonstrate that the restriction of productive VZV infection in CHO-K1 cells occurs downstream of virus entry. Entry of VZV into CHO-K1 cells was characterized by utilizing an ICP4/beta-galactosidase reporter gene that has been used previously to study herpes simplex virus type 1 entry. Entry of VZV into CHO-K1 cells involved cell surface interactions with heparan sulfate glycosaminoglycans and a cation-independent mannose-6-phosphate receptor. Lysosomotropic agents inhibited the entry of VZV into CHO-K1 cells, consistent with a low-pH-dependent endocytic mechanism of entry. Infection of CHO-K1 cells by VZV resulted in the production of both immediate early and late gene products, indicating that a block to progeny virus production occurs after the initiation of virus gene expression.
Figures

Similar articles
-
Soluble 3-O-sulfated heparan sulfate can trigger herpes simplex virus type 1 entry into resistant Chinese hamster ovary (CHO-K1) cells.J Gen Virol. 2007 Apr;88(Pt 4):1075-1079. doi: 10.1099/vir.0.82476-0. J Gen Virol. 2007. PMID: 17374750
-
The varicella-zoster virus-mediated delayed host shutoff: open reading frame 17 has no major function, whereas immediate-early 63 protein represses heterologous gene expression.Microbes Infect. 2005 Dec;7(15):1519-29. doi: 10.1016/j.micinf.2005.05.010. Epub 2005 Jul 1. Microbes Infect. 2005. PMID: 16039898
-
Productive vs non-productive infection by cell-free varicella zoster virus of human neurons derived from embryonic stem cells is dependent upon infectious viral dose.Virology. 2013 Sep 1;443(2):285-93. doi: 10.1016/j.virol.2013.05.021. Epub 2013 Jun 12. Virology. 2013. PMID: 23769240 Free PMC article.
-
[Varicella-zoster virus (VZV)].Uirusu. 2010 Dec;60(2):197-207. doi: 10.2222/jsv.60.197. Uirusu. 2010. PMID: 21488333 Review. Japanese.
-
Varicella-zoster virus: molecular virology and virus-host interactions.Curr Opin Microbiol. 2001 Aug;4(4):442-9. doi: 10.1016/s1369-5274(00)00233-2. Curr Opin Microbiol. 2001. PMID: 11495809 Review.
Cited by
-
A structure-based approach for mapping adverse drug reactions to the perturbation of underlying biological pathways.PLoS One. 2010 Aug 23;5(8):e12063. doi: 10.1371/journal.pone.0012063. PLoS One. 2010. PMID: 20808786 Free PMC article.
-
A Guide to Preclinical Models of Zoster-Associated Pain and Postherpetic Neuralgia.Curr Top Microbiol Immunol. 2023;438:189-221. doi: 10.1007/82_2021_240. Curr Top Microbiol Immunol. 2023. PMID: 34524508 Review.
-
Most rotavirus strains require the cation-independent mannose-6-phosphate receptor, sortilin-1, and cathepsins to enter cells.Virus Res. 2018 Feb 2;245:44-51. doi: 10.1016/j.virusres.2017.12.002. Epub 2017 Dec 21. Virus Res. 2018. PMID: 29275103 Free PMC article.
-
Varicella zoster virus encodes a viral decoy RHIM to inhibit cell death.PLoS Pathog. 2020 Jul 10;16(7):e1008473. doi: 10.1371/journal.ppat.1008473. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32649716 Free PMC article.
-
Herpes Simplex Virus Glycoprotein C Regulates Low-pH Entry.mSphere. 2020 Feb 5;5(1):e00826-19. doi: 10.1128/mSphere.00826-19. mSphere. 2020. PMID: 32024702 Free PMC article.
References
-
- Annunziato, P., P. LaRussa, P. Lee, S. Steinberg, O. Lungu, A. A. Gershon, and S. Silverstein. 1998. Evidence of latent varicella-zoster virus in rat dorsal root ganglia. J. Infect. Dis. 178(Suppl. 1):S48-S51. - PubMed
-
- Banfield, B. W., Y. Leduc, L. Esford, R. J. Visalli, C. R. Brandt, and F. Tufaro. 1995. Evidence for an interaction of herpes simplex virus with chondroitin sulfate proteoglycans during infection. Virology 208:531-539. - PubMed
-
- Bourdon-Wouters, C., M. P. Merville-Louis, C. Sadzot-Delvaux, P. Marc, J. Piette, P. Delree, G. Moonen, and B. Rentier. 1990. Acute and persistent varicella-zoster virus infection of human and murine neuroblastoma cell lines. J. Neurosci. Res. 26:90-97. - PubMed
-
- Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol. 82:1419-1422. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

