BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo
- PMID: 17040210
- PMCID: PMC1698666
- DOI: 10.1042/BJ20061088
BI-D1870 is a specific inhibitor of the p90 RSK (ribosomal S6 kinase) isoforms in vitro and in vivo
Abstract
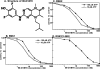
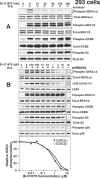
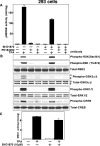
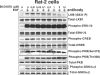
Hormones and growth factors induce the activation of a number of protein kinases that belong to the AGC subfamily, including isoforms of PKA, protein kinase B (also known as Akt), PKC, S6K p70 (ribosomal S6 kinase), RSK (p90 ribosomal S6 kinase) and MSK (mitogen- and stress-activated protein kinase), which then mediate many of the physiological processes that are regulated by these extracellular agonists. It can be difficult to assess the individual functions of each AGC kinase because their substrate specificities are similar. Here we describe the small molecule BI-D1870, which inhibits RSK1, RSK2, RSK3 and RSK4 in vitro with an IC(50) of 10-30 nM, but does not signi-ficantly inhibit ten other AGC kinase members and over 40 other protein kinases tested at 100-fold higher concentrations. BI-D1870 is cell permeant and prevents the RSK-mediated phorbol ester- and EGF (epidermal growth factor)-induced phosphoryl-ation of glycogen synthase kinase-3beta and LKB1 in human embry-onic kidney 293 cells and Rat-2 cells. In contrast, BI-D1870 does not affect the agonist-triggered phosphorylation of substrates for six other AGC kinases. Moreover, BI-D1870 does not suppress the phorbol ester- or EGF-induced phosphorylation of CREB (cAMP-response-element-binding protein), consistent with the genetic evidence indicating that MSK, and not RSK, isoforms mediate the mitogen-induced phosphorylation of this transcription factor.
Figures



Comment in
-
A sharper instrument for dissecting signalling events: a specific AGC kinase inhibitor.Biochem J. 2007 Jan 1;401(1):e1-3. doi: 10.1042/BJ20061691. Biochem J. 2007. PMID: 17150039 Free PMC article.
Similar articles
-
Two widely used RSK inhibitors, BI-D1870 and SL0101, alter mTORC1 signaling in a RSK-independent manner.Cell Signal. 2015 Aug;27(8):1630-42. doi: 10.1016/j.cellsig.2015.04.004. Epub 2015 Apr 16. Cell Signal. 2015. PMID: 25889895
-
The p90 ribosomal S6 kinase (RSK) inhibitor BI-D1870 prevents gamma irradiation-induced apoptosis and mediates senescence via RSK- and p53-independent accumulation of p21WAF1/CIP1.Cell Death Dis. 2013 Oct 17;4(10):e859. doi: 10.1038/cddis.2013.386. Cell Death Dis. 2013. PMID: 24136223 Free PMC article.
-
Overcoming resistance to Sonic Hedgehog inhibition by targeting p90 ribosomal S6 kinase in pediatric medulloblastoma.Pediatr Blood Cancer. 2014 Jan;61(1):107-15. doi: 10.1002/pbc.24675. Epub 2013 Aug 12. Pediatr Blood Cancer. 2014. PMID: 23940083
-
The RSK factors of activating the Ras/MAPK signaling cascade.Front Biosci. 2008 May 1;13:4258-75. doi: 10.2741/3003. Front Biosci. 2008. PMID: 18508509 Review.
-
Regulation and function of the RSK family of protein kinases.Biochem J. 2012 Jan 15;441(2):553-69. doi: 10.1042/BJ20110289. Biochem J. 2012. PMID: 22187936 Review.
Cited by
-
The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome.Cell Host Microbe. 2016 Sep 14;20(3):296-306. doi: 10.1016/j.chom.2016.07.018. Epub 2016 Aug 25. Cell Host Microbe. 2016. PMID: 27569559 Free PMC article.
-
PDCD4 limits prooncogenic neuregulin-ErbB signaling.Cell Mol Life Sci. 2021 Feb;78(4):1799-1815. doi: 10.1007/s00018-020-03617-5. Epub 2020 Aug 17. Cell Mol Life Sci. 2021. PMID: 32804243 Free PMC article.
-
p90RSK pathway inhibition synergizes with cisplatin in TMEM16A overexpressing head and neck cancer.BMC Cancer. 2024 Feb 19;24(1):233. doi: 10.1186/s12885-024-11892-9. BMC Cancer. 2024. PMID: 38373988 Free PMC article.
-
The Coffin-Lowry syndrome-associated protein RSK2 regulates neurite outgrowth through phosphorylation of phospholipase D1 (PLD1) and synthesis of phosphatidic acid.J Neurosci. 2013 Dec 11;33(50):19470-9. doi: 10.1523/JNEUROSCI.2283-13.2013. J Neurosci. 2013. PMID: 24336713 Free PMC article.
-
A Rising Cancer Prevention Target of RSK2 in Human Skin Cancer.Front Oncol. 2013 Aug 5;3:201. doi: 10.3389/fonc.2013.00201. eCollection 2013. Front Oncol. 2013. PMID: 23936765 Free PMC article.
References
-
- Scott J. D. Cyclic nucleotide-dependent protein kinases. Pharmacol. Therapeut. 1991;50:123–145. - PubMed
-
- Avruch J., Belham C., Weng Q., Hara K., Yonezawa K. The p70 S6 kinase integrates nutrient and growth signals to control translational capacity. Prog. Mol. Subcell. Biol. 2001;26:115–154. - PubMed
-
- Dalby K. N., Morrice N., Caudwell F. B., Avruch J., Cohen P. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 1998;273:1496–1505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

