Human cytomegalovirus infection alters PC3 prostate carcinoma cell adhesion to endothelial cells and extracellular matrix
- PMID: 17032497
- PMCID: PMC1715925
- DOI: 10.1593/neo.06379
Human cytomegalovirus infection alters PC3 prostate carcinoma cell adhesion to endothelial cells and extracellular matrix
Abstract
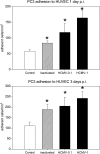
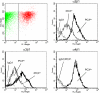
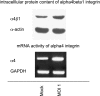
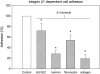
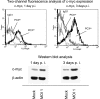
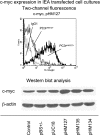
The genome and antigens of human cytomegalovirus (HCMV) are frequently found in prostatic carcinoma. However, whether this infection is causative or is an epiphenomenon is not clear. We therefore investigated the ability of HCMV to promote metastatic processes, defined by tumor cell adhesion to the endothelium and extracellular matrix proteins. Experiments were based on the human prostate tumor cell line PC3, either infected with the HCMV strain Hi (HCMV(Hi)) or transfected with cDNA encoding the HCMV-specific immediate early protein IEA1 (UL123) or IEA2 (UL122). HCMV(Hi) upregulated PC3 adhesion to the endothelium and to the extracellular matrix proteins collagen, laminin, and fibronectin. The process was accompanied by enhancement of beta(1)-integrin surface expression, elevated levels of integrin-linked kinase, and phosphorylation of focal adhesion kinase. IEA1 or IEA2 did not modulate PC3 adhesion or beta(1)-integrin expression. Based on this in vitro model, we postulate a direct association between HCMV infection and prostate tumor transmigration, which is not dependent on IEA proteins. Integrin overexpression, combined with the modulation of integrin-dependent signalling, seems to be, at least in part, responsible for a more invasive PC3(Hi) tumor cell phenotype. Elevated levels of c-myc found in IEA1-transfected or IEA2-transfected PC3 cell populations might promote further carcinogenic processes through accelerated cell proliferation.
Figures



Similar articles
-
High dose fractionated ionizing radiation inhibits prostate cancer cell adhesion and beta(1) integrin expression.Prostate. 2005 Jun 15;64(1):83-91. doi: 10.1002/pros.20227. Prostate. 2005. PMID: 15651037
-
Secreted frizzled-related protein 4 inhibits proliferation and metastatic potential in prostate cancer.Prostate. 2007 Jul 1;67(10):1081-90. doi: 10.1002/pros.20607. Prostate. 2007. PMID: 17476687
-
Human cytomegalovirus infection of tumor cells downregulates NCAM (CD56): a novel mechanism for virus-induced tumor invasiveness.Neoplasia. 2004 Jul-Aug;6(4):323-31. doi: 10.1593/neo.03418. Neoplasia. 2004. PMID: 15256054 Free PMC article.
-
Oncomodulatory signals by regulatory proteins encoded by human cytomegalovirus: a novel role for viral infection in tumor progression.FEMS Microbiol Rev. 2004 Feb;28(1):59-77. doi: 10.1016/j.femsre.2003.07.005. FEMS Microbiol Rev. 2004. PMID: 14975530 Review.
-
An intracellular signal pathway that regulates cancer cell adhesion in response to extracellular forces.Cancer Res. 2008 Jan 1;68(1):2-4. doi: 10.1158/0008-5472.CAN-07-2992. Cancer Res. 2008. PMID: 18172287 Review.
Cited by
-
Human Cytomegalovirus interleukin-10 promotes proliferation and migration of MCF-7 breast cancer cells.Cancer Cell Microenviron. 2015;2(1):e678. doi: 10.14800/ccm.678. Cancer Cell Microenviron. 2015. PMID: 26023679 Free PMC article.
-
Human cytomegalovirus infection enhances NF-κB/p65 signaling in inflammatory breast cancer patients.PLoS One. 2013;8(2):e55755. doi: 10.1371/journal.pone.0055755. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418456 Free PMC article.
-
cmvIL-10 stimulates the invasive potential of MDA-MB-231 breast cancer cells.PLoS One. 2014 Feb 10;9(2):e88708. doi: 10.1371/journal.pone.0088708. eCollection 2014. PLoS One. 2014. PMID: 24520416 Free PMC article.
-
Polyploid Giant Cancer Cells Generated from Human Cytomegalovirus-Infected Prostate Epithelial Cells.Cancers (Basel). 2023 Oct 15;15(20):4994. doi: 10.3390/cancers15204994. Cancers (Basel). 2023. PMID: 37894361 Free PMC article.
-
Neoplasia: the second decade.Neoplasia. 2008 Dec;10(12):1314-24. doi: 10.1593/neo.81372. Neoplasia. 2008. PMID: 19048110 Free PMC article.
References
-
- Cinatl J, Scholz M, Kotchetkov R, Vogel JU, Doerr HW. Molecular mechanisms of the modulatory effects of HCMV infection in tumor cell biology. Trends Mol Med. 2004;10:19–23. - PubMed
-
- Nomura AM, Kolonel LN. Prostate cancer: a current perspective. Epidemiol Rev. 1991;13:200–227. - PubMed
-
- Quinn M, Babb P. Patterns and trends in prostate cancer incidence, survival, prevalence and mortality: Part II. Individual countries. BJU Int. 2002;90:174–184. - PubMed
-
- Dennis LK, Dawson DV. Meta-analysis of measures of sexual activity and prostate cancer. Epidemiology. 2002;13:72–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
