Anti-tumor effect in an in vivo model by human-derived pancreatic RNase with basic fibroblast growth factor insertional fusion protein through antiangiogenic properties
- PMID: 17032310
- PMCID: PMC11158387
- DOI: 10.1111/j.1349-7006.2006.00336.x
Anti-tumor effect in an in vivo model by human-derived pancreatic RNase with basic fibroblast growth factor insertional fusion protein through antiangiogenic properties
Abstract
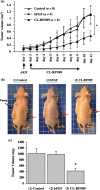
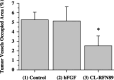
It is thought that the export of angiogenic fibroblast growth factors (FGF) from tumors may be involved in the onset of tumor angiogenesis. To create a new active targeting drug that inhibits the tumor angiogenic process without toxicities to normal cells, human basic FGF (h-bFGF) was inserted genetically into the Gly89 position of cross-linked RNase1 (the ribonuclease inhibitor protein [RI] binding site of cross-linked human pancreatic RNase) to prevent stereospecific binding to RI. The resultant insertional-fusion protein (CL-RFN89) was active both as h-bFGF and as RNase1. Furthermore, it acquired an additional ability of evading RI through steric blockade of RI binding caused by the fused h-bFGF domain. In the present study, the effect of the resultant protein, CL-RFN89, on the antitumor response though its antiangiogenic properties was investigated in an in vivo model. Continuous systemic treatment with CL-RFN89 significantly inhibited the growth of human A431 squamous cell carcinomas in vivo. Seven days of treatment with CL-RFN89 resulted in a 58.2% inhibition of tumor growth compared with control mice (P < 0.0001). Furthermore, immunohistochemistry using a rat antimouse CD31 antibody showed that treatment with CL-RFN89 reduced tumor vascularization. These findings identify CL-RFN89 as a potent systemic inhibitor of tumor growth as a result of its antiangiogenic properties. This protein appears to be a new systemic antitumor agent.
Figures

Similar articles
-
Insertional-fusion of basic fibroblast growth factor endowed ribonuclease 1 with enhanced cytotoxicity by steric blockade of inhibitor interaction.FEBS Lett. 2004 Jun 18;568(1-3):39-43. doi: 10.1016/j.febslet.2004.05.007. FEBS Lett. 2004. PMID: 15196917
-
Anti-angiogenic effect of an insertional fusion protein of human basic fibroblast growth factor and ribonuclease-1.Protein Eng Des Sel. 2005 Jul;18(7):321-7. doi: 10.1093/protein/gzi040. Epub 2005 Jun 24. Protein Eng Des Sel. 2005. PMID: 15980015
-
Inhibition of angiogenesis and angiogenesis-dependent tumor growth by the cryptic kringle fragments of human apolipoprotein(a).J Biol Chem. 2003 Aug 1;278(31):29000-8. doi: 10.1074/jbc.M301042200. Epub 2003 May 13. J Biol Chem. 2003. PMID: 12746434
-
Dual blockade of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (FGF-2) exhibits potent anti-angiogenic effects.Cancer Lett. 2016 Jul 28;377(2):164-73. doi: 10.1016/j.canlet.2016.04.036. Epub 2016 Apr 26. Cancer Lett. 2016. PMID: 27130666
-
Targeting tumor micro-environment for design and development of novel anti-angiogenic agents arresting tumor growth.Prog Biophys Mol Biol. 2013 Nov;113(2):333-54. doi: 10.1016/j.pbiomolbio.2013.10.001. Epub 2013 Oct 15. Prog Biophys Mol Biol. 2013. PMID: 24139944 Review.
Cited by
-
Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity.Curr Pharm Biotechnol. 2008 Jun;9(3):185-9. doi: 10.2174/138920108784567344. Curr Pharm Biotechnol. 2008. PMID: 18673284 Free PMC article. Review.
-
Endothelial Ribonuclease 1 in Cardiovascular and Systemic Inflammation.Front Cell Dev Biol. 2020 Sep 4;8:576491. doi: 10.3389/fcell.2020.576491. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015070 Free PMC article. Review.
References
-
- Lappi DA, Baird A. Mitotoxins: growth factor‐targeted cytotoxic molecules. Prog Growth Factor Res 1990; 2: 223–36. - PubMed
-
- Pastan II, Kreitman RJ. Immunotoxins for targeted cancer therapy. Adv Drug Deliv Rev 1998; 31: 53–88. - PubMed
-
- Folkman J. New perspectives in clinical oncology from angiogenesis research. Eur J Cancer 1996; 32: 2534–9. - PubMed
-
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell 1996; 86: 353–64. - PubMed
-
- Hanahan D, Christofori G, Naik P, Arbeit J. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer 1996; 32: 2386–93. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

