Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis
- PMID: 17015472
- PMCID: PMC1636857
- DOI: 10.1128/MCB.02282-05
Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis
Abstract
Apoptosis, induced by a number of death stimuli, is associated with a fragmentation of the mitochondrial network. These morphological changes in mitochondria have been shown to require proteins, such as Drp1 or hFis1, which are involved in regulating the fission of mitochondria. However, the precise role of mitochondrial fission during apoptosis remains elusive. Here we report that inhibiting the fission machinery in Bax/Bak-mediated apoptosis, by down-regulating of Drp1 or hFis1, prevents the fragmentation of the mitochondrial network and partially inhibits the release of cytochrome c from the mitochondria but fails to block the efflux of Smac/DIABLO. In addition, preventing mitochondrial fragmentation does not inhibit cell death induced by Bax/Bak-dependent death stimuli, in contrast to the effects of Bcl-xL or caspase inhibition. Therefore, the fission of mitochondria is a dispensable event in Bax/Bak-dependent apoptosis.
Figures

 , P < 0.05;
, P < 0.05; 
 , P < 0.005;
, P < 0.005; 

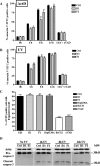
 , P < 0.0005; ns, not significant). Panel B presents data obtained as described for panel A except that apoptosis was triggered by UV irradiation.
, P < 0.0005; ns, not significant). Panel B presents data obtained as described for panel A except that apoptosis was triggered by UV irradiation. 
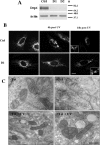
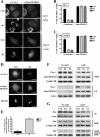
 , P < 0.005; ns, not significant. (C) Cos-7 cells were cotransfected with pEYFPmito and control, D1, or F1 shRNA constructs or pCiDrpK38A or pCiBcl-Xl; 72 h after transfection, the cells were treated with ActD (6 μM) for 14 h and just before collection the cells were incubated with propidium iodide and Hoechst 33342 for 15 min. The histogram represents a quantitative analysis of the percentage of the propidium iodide-negative Cos-7 cells expressing yellow fluorescent protein (YFP) that displayed apoptotic nuclei as observed by Hoechst 33342 staining. The data are expressed as means + SEMs of the results of three independent experiments.
, P < 0.005; ns, not significant. (C) Cos-7 cells were cotransfected with pEYFPmito and control, D1, or F1 shRNA constructs or pCiDrpK38A or pCiBcl-Xl; 72 h after transfection, the cells were treated with ActD (6 μM) for 14 h and just before collection the cells were incubated with propidium iodide and Hoechst 33342 for 15 min. The histogram represents a quantitative analysis of the percentage of the propidium iodide-negative Cos-7 cells expressing yellow fluorescent protein (YFP) that displayed apoptotic nuclei as observed by Hoechst 33342 staining. The data are expressed as means + SEMs of the results of three independent experiments.

 , P < 0.0005; ns, not significant. (D) HeLa cells were treated as described for panel B, and the cleavage of caspase-3 was assessed by Western blotting at the indicated times.
, P < 0.0005; ns, not significant. (D) HeLa cells were treated as described for panel B, and the cleavage of caspase-3 was assessed by Western blotting at the indicated times.Similar articles
-
Bax/Bak-dependent, Drp1-independent Targeting of X-linked Inhibitor of Apoptosis Protein (XIAP) into Inner Mitochondrial Compartments Counteracts Smac/DIABLO-dependent Effector Caspase Activation.J Biol Chem. 2015 Sep 4;290(36):22005-18. doi: 10.1074/jbc.M115.643064. Epub 2015 Jul 1. J Biol Chem. 2015. PMID: 26134559 Free PMC article.
-
Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death.J Cell Biol. 2007 May 7;177(3):439-50. doi: 10.1083/jcb.200610042. Epub 2007 Apr 30. J Cell Biol. 2007. PMID: 17470634 Free PMC article.
-
Drp1 is dispensable for apoptotic cytochrome c release in primed MCF10A and fibroblast cells but affects Bcl-2 antagonist-induced respiratory changes.Br J Pharmacol. 2014 Apr;171(8):1988-99. doi: 10.1111/bph.12515. Br J Pharmacol. 2014. PMID: 24206264 Free PMC article.
-
Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane.Biochim Biophys Acta Mol Cell Res. 2022 Oct;1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317. Epub 2022 Jun 22. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35752202 Review.
-
Regulated necrotic cell death: the passive aggressive side of Bax and Bak.Circ Res. 2015 May 22;116(11):1800-9. doi: 10.1161/CIRCRESAHA.116.305421. Circ Res. 2015. PMID: 25999420 Free PMC article. Review.
Cited by
-
The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice.J Cell Biol. 2009 Sep 21;186(6):805-16. doi: 10.1083/jcb.200903065. Epub 2009 Sep 14. J Cell Biol. 2009. PMID: 19752021 Free PMC article.
-
Cdk8 Kinase Module: A Mediator of Life and Death Decisions in Times of Stress.Microorganisms. 2021 Oct 15;9(10):2152. doi: 10.3390/microorganisms9102152. Microorganisms. 2021. PMID: 34683473 Free PMC article. Review.
-
Death on the slopes. Symposium on Cell Death Pathways.EMBO Rep. 2009 Aug;10(8):827-31. doi: 10.1038/embor.2009.160. Epub 2009 Jul 17. EMBO Rep. 2009. PMID: 19609322 Free PMC article. Review. No abstract available.
-
Mitochondrial-Shaping Proteins in Cardiac Health and Disease - the Long and the Short of It!Cardiovasc Drugs Ther. 2017 Feb;31(1):87-107. doi: 10.1007/s10557-016-6710-1. Cardiovasc Drugs Ther. 2017. PMID: 28190190 Free PMC article. Review.
-
Stress-Activated Degradation of Sphingolipids Regulates Mitochondrial Function and Cell Death in Yeast.Oxid Med Cell Longev. 2017;2017:2708345. doi: 10.1155/2017/2708345. Epub 2017 Aug 6. Oxid Med Cell Longev. 2017. PMID: 28845213 Free PMC article.
References
-
- Arnoult, D., A. Grodet, Y. J. Lee, J. Estaquier, and C. Blackstone. 2005. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J. Biol. Chem. 280:35742-35750. - PubMed
-
- Bernardi, P., and G. F. Azzone. 1981. Cytochrome c as an electron shuttle between the outer and inner mitochondrial membranes. J. Biol. Chem. 256:7187-7192. - PubMed
-
- Bossy-Wetzel, E., M. J. Barsoum, A. Godzik, R. Schwarzenbacher, and S. A. Lipton. 2003. Mitochondrial fission in apoptosis, neurodegeneration and aging. Curr. Opin. Cell Biol. 15:706-716. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
