In vitro and cellular assays for palmitoyl acyltransferases using fluorescent lipidated peptides
- PMID: 17012028
- PMCID: PMC2892119
- DOI: 10.1016/j.ymeth.2006.06.019
In vitro and cellular assays for palmitoyl acyltransferases using fluorescent lipidated peptides
Abstract
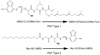
Protein palmitoylation is emerging as an important post-translational modification in development as well as in the establishment and progression of diseases such as cancer. This chapter describes the use of fluorescent lipidated peptides to characterize palmitoyl acyltransferase (PAT) activities in vitro and in intact cells. The peptides mimic two motifs that are enzymatically palmitoylated, i.e. C-terminal farnesyl and N-terminal myristoyl sequences. These substrate peptides can be separated from the palmitoylated product peptides by reversed-phase HPLC, detected and quantified by the fluorescence of their NBD label. Through these methods, the activities of PATs toward these alternate substrates in isolated membranes or intact cells can be quantified. The in vitro assay has been used to characterize human PATs and to identify inhibitors of these enzymes. The cellular assay has been useful in elucidating the kinetics of protein palmitoylation by PATs in situ, and the sub-cellular distribution of the palmitoylated products.
Figures


Similar articles
-
Cellular palmitoylation and trafficking of lipidated peptides.J Lipid Res. 2007 Aug;48(8):1873-84. doi: 10.1194/jlr.M700179-JLR200. Epub 2007 May 24. J Lipid Res. 2007. PMID: 17525474 Free PMC article.
-
Characterization of human palmitoyl-acyl transferase activity using peptides that mimic distinct palmitoylation motifs.Biochem J. 2003 Jul 1;373(Pt 1):91-9. doi: 10.1042/BJ20021598. Biochem J. 2003. PMID: 12670300 Free PMC article.
-
Sensitive and rapid analysis of protein palmitoylation with a synthetic cell-permeable mimic of SRC oncoproteins.J Am Chem Soc. 2002 Mar 20;124(11):2444-5. doi: 10.1021/ja017671x. J Am Chem Soc. 2002. PMID: 11890786
-
Purification and characterization of recombinant protein acyltransferases.Methods. 2006 Oct;40(2):143-50. doi: 10.1016/j.ymeth.2006.07.017. Methods. 2006. PMID: 17012026 Free PMC article. Review.
-
Emerging Roles of DHHC-mediated Protein S-palmitoylation in Physiological and Pathophysiological Context.Eur J Cell Biol. 2018 Jun;97(5):319-338. doi: 10.1016/j.ejcb.2018.03.005. Epub 2018 Mar 22. Eur J Cell Biol. 2018. PMID: 29602512 Review.
Cited by
-
Use of micellar electrokinetic chromatography to measure palmitoylation of a peptide.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Nov 15;875(2):451-8. doi: 10.1016/j.jchromb.2008.09.026. Epub 2008 Sep 30. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18926781 Free PMC article.
-
Recent advances in chemical proteomics: exploring the post-translational proteome.J Chem Biol. 2008 Nov;1(1-4):17-26. doi: 10.1007/s12154-008-0002-6. Epub 2008 May 9. J Chem Biol. 2008. PMID: 19568795 Free PMC article.
-
Regulation of Dynamic Protein S-Acylation.Front Mol Biosci. 2021 Apr 26;8:656440. doi: 10.3389/fmolb.2021.656440. eCollection 2021. Front Mol Biosci. 2021. PMID: 33981723 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

