Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles
- PMID: 17005647
- PMCID: PMC1642584
- DOI: 10.1128/JVI.01717-06
Hepatitis C virus entry requires a critical postinternalization step and delivery to early endosomes via clathrin-coated vesicles
Abstract
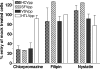
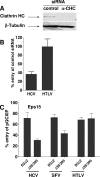
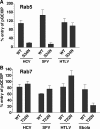
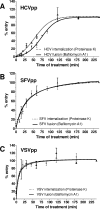
Hepatitis C virus (HCV) is a major human pathogen associated with life-threatening liver disease. Entry into hepatocytes requires CD81 and a putative second receptor. In this study, we elucidated the postreceptor attachment stages of HCV entry using HCV pseudoparticles (HCVpp) as a model system. By means of dominant-negative mutants and short interfering RNAs of various cellular proteins, we showed that HCVpp enter via clathrin-coated vesicles and require delivery to early but not to late endosomes. However, the kinetics of HCV envelope glycoprotein-mediated fusion are delayed compared to those of other viruses that enter in early endosomes. Entry of HCVpp can be efficiently blocked by bafilomycin A1, which neutralizes the pH in early endosomes and impairs progression of endocytosis beyond this stage. However, low-pH exposure of bafilomycin A1-treated target cells does not induce entry of HCVpp at the plasma membrane or in the early stages of endocytosis. These observations indicate that, subsequent to internalization, HCVpp entry necessitates additional, low-pH-dependent interactions, modifications, or trafficking, and that these events are irreversibly disrupted by bafilomycin A1 treatment.
Figures
Similar articles
-
Hepatitis C virus entry depends on clathrin-mediated endocytosis.J Virol. 2006 Jul;80(14):6964-72. doi: 10.1128/JVI.00024-06. J Virol. 2006. PMID: 16809302 Free PMC article.
-
Arbidol inhibits viral entry by interfering with clathrin-dependent trafficking.Antiviral Res. 2013 Oct;100(1):215-9. doi: 10.1016/j.antiviral.2013.08.008. Epub 2013 Aug 25. Antiviral Res. 2013. PMID: 23981392
-
Actin dynamics regulate multiple endosomal steps during Kaposi's sarcoma-associated herpesvirus entry and trafficking in endothelial cells.PLoS Pathog. 2009 Jul;5(7):e1000512. doi: 10.1371/journal.ppat.1000512. Epub 2009 Jul 10. PLoS Pathog. 2009. PMID: 19593382 Free PMC article.
-
The hepatitis C virus and its hepatic environment: a toxic but finely tuned partnership.Biochem J. 2009 Oct 12;423(3):303-14. doi: 10.1042/BJ20091000. Biochem J. 2009. PMID: 19807698 Review.
-
Interaction of KSHV with host cell surface receptors and cell entry.Viruses. 2014 Oct 23;6(10):4024-46. doi: 10.3390/v6104024. Viruses. 2014. PMID: 25341665 Free PMC article. Review.
Cited by
-
Flunarizine prevents hepatitis C virus membrane fusion in a genotype-dependent manner by targeting the potential fusion peptide within E1.Hepatology. 2016 Jan;63(1):49-62. doi: 10.1002/hep.28111. Epub 2015 Oct 9. Hepatology. 2016. PMID: 26248546 Free PMC article.
-
Mouse-specific residues of claudin-1 limit hepatitis C virus genotype 2a infection in a human hepatocyte cell line.J Virol. 2010 Jan;84(2):964-75. doi: 10.1128/JVI.01504-09. Epub 2009 Nov 4. J Virol. 2010. PMID: 19889758 Free PMC article.
-
Hijacking the endocytic machinery by microbial pathogens.Protoplasma. 2010 Aug;244(1-4):75-90. doi: 10.1007/s00709-010-0164-2. Epub 2010 Jun 25. Protoplasma. 2010. PMID: 20574860 Review.
-
Identification of GBF1 as a cellular factor required for hepatitis C virus RNA replication.J Virol. 2010 Jan;84(2):773-87. doi: 10.1128/JVI.01190-09. Epub 2009 Nov 11. J Virol. 2010. PMID: 19906930 Free PMC article.
-
Direct infection and replication of naturally occurring hepatitis C virus genotypes 1, 2, 3 and 4 in normal human hepatocyte cultures.PLoS One. 2008 Jul 16;3(7):e2660. doi: 10.1371/journal.pone.0002660. PLoS One. 2008. PMID: 18628977 Free PMC article.
References
-
- Baravalle, G., D. Schober, M. Huber, N. Bayer, R. F. Murphy, and R. Fuchs. 2005. Transferrin recycling and dextran transport to lysosomes is differentially affected by bafilomycin, nocodazole, and low temperature. Cell Tissue Res. 320:99-113. - PubMed
-
- Barbieri, M. A., G. Li, M. I. Colombo, and P. D. Stahl. 1994. Rab5, an early acting endosomal GTPase, supports in vitro endosome fusion without GTP hydrolysis. J. Biol. Chem. 269:18720-18722. - PubMed
-
- Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F. L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 278:41624-41630. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

