A specific survival response in dopamine neurons at most risk in Parkinson's disease
- PMID: 16988046
- PMCID: PMC6674460
- DOI: 10.1523/JNEUROSCI.2745-06.2006
A specific survival response in dopamine neurons at most risk in Parkinson's disease
Abstract
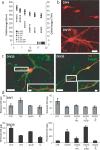
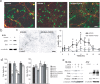
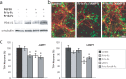
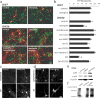
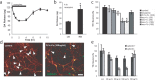
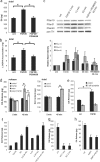
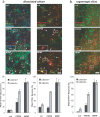
The specific expression of fibroblast growth factor 20 (FGF-20) in the adult substantia nigra and the association between FGF-20 mutations and Parkinson's disease provoked exploration of the function of this growth factor. We show by gain- and loss-of-function in vitro experiments that FGF-20 promotes survival and stimulates dopamine (DA) release in a calbindin-negative subset of cells that are preferentially lost in Parkinson's disease. FGF-20 selectively activates tyrosine hydroxylase in calbindin-negative neurons. In the adult substantia nigra, calbindin-negative neurons specifically express high levels of FGFR1 (FGF receptor 1). These data show that FGF signals to elevate DA levels and protect the specific midbrain neuron type at most risk in Parkinson's patients.
Figures

Similar articles
-
The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system.Brain Res Rev. 2007 Apr;54(1):80-91. doi: 10.1016/j.brainresrev.2006.12.001. Epub 2007 Jan 16. Brain Res Rev. 2007. PMID: 17229467 Review.
-
The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease.Brain. 1999 Aug;122 ( Pt 8):1437-48. doi: 10.1093/brain/122.8.1437. Brain. 1999. PMID: 10430830
-
Preferential neurotrophic activity of fibroblast growth factor-20 for dopaminergic neurons through fibroblast growth factor receptor-1c.J Neurosci Res. 2003 May 15;72(4):436-43. doi: 10.1002/jnr.10592. J Neurosci Res. 2003. PMID: 12704805
-
Is Bax a mitochondrial mediator in apoptotic death of dopaminergic neurons in Parkinson's disease?J Neurochem. 2001 Mar;76(6):1785-93. doi: 10.1046/j.1471-4159.2001.00160.x. J Neurochem. 2001. PMID: 11259496
-
Neurotrophic support of midbrain dopaminergic neurons.Adv Exp Med Biol. 2009;651:73-80. doi: 10.1007/978-1-4419-0322-8_7. Adv Exp Med Biol. 2009. PMID: 19731552 Review.
Cited by
-
Roles of FGF20 in dopaminergic neurons and Parkinson's disease.Front Mol Neurosci. 2013 May 31;6:15. doi: 10.3389/fnmol.2013.00015. eCollection 2013. Front Mol Neurosci. 2013. PMID: 23754977 Free PMC article.
-
Molecular pathology of the fibroblast growth factor family.Hum Mutat. 2009 Sep;30(9):1245-55. doi: 10.1002/humu.21067. Hum Mutat. 2009. PMID: 19621416 Free PMC article. Review.
-
Repeated diagnostic ultrasound exposure modifies the structural properties of CA1 dendrites and alters the hippocampal transcriptome.Sci Rep. 2024 May 22;14(1):11713. doi: 10.1038/s41598-024-62621-y. Sci Rep. 2024. PMID: 38778177 Free PMC article.
-
Mechanisms by which fibroblast growth factor 20 improves motor performance in a mouse model of Parkinson's disease.Neural Regen Res. 2019 Aug;14(8):1438-1444. doi: 10.4103/1673-5374.253527. Neural Regen Res. 2019. PMID: 30964070 Free PMC article.
-
Quercetin mitigates the deoxynivalenol mycotoxin induced apoptosis in SH-SY5Y cells by modulating the oxidative stress mediators.Saudi J Biol Sci. 2021 Jan;28(1):465-477. doi: 10.1016/j.sjbs.2020.10.030. Epub 2020 Oct 27. Saudi J Biol Sci. 2021. PMID: 33424329 Free PMC article.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A. Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. - PubMed
-
- Acsadi G, Anguelov RA, Yang H, Toth G, Thomas R, Jani A, Wang Y, Ianakova E, Mohammad S, Lewis RA, Shy ME. Increased survival and function of SOD1 mice after glial cell-derived neurotrophic factor gene therapy. Hum Gene Ther. 2002;13:1047–1059. - PubMed
-
- Adams JD, Jr, Chang ML, Klaidman L. Parkinson's disease-redox mechanisms. Curr Med Chem. 2001;8:809–814. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

