Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon
- PMID: 16985170
- PMCID: PMC8254533
- DOI: 10.1182/blood-2006-05-023770
Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon
Abstract
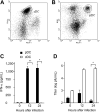
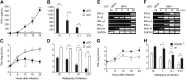
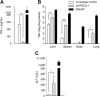
This study demonstrates a unique and crucial role of plasmacytoid dendritic cells (pDCs) and pDC-derived type I interferons (IFNs) in the pathogenesis of mouse coronavirus infection. pDCs controlled the fast replicating mouse hepatitis virus (MHV) through the immediate production of type I IFNs. Recognition of MHV by pDCs was mediated via TLR7 ensuring a swift IFN-alpha production following encounter with this cytopathic RNA virus. Furthermore, the particular type I IFN response pattern was not restricted to the murine coronavirus, but was also found in infection with the highly cytopathic human severe acute respiratory syndrome (SARS) coronavirus. Taken together, our results suggest that rapid production of type I IFNs by pDCs is essential for the control of potentially lethal coronavirus infections.
Figures



Similar articles
-
High secretion of interferons by human plasmacytoid dendritic cells upon recognition of Middle East respiratory syndrome coronavirus.J Virol. 2015 Apr;89(7):3859-69. doi: 10.1128/JVI.03607-14. Epub 2015 Jan 21. J Virol. 2015. PMID: 25609809 Free PMC article.
-
Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection.J Immunol. 2009 Jan 15;182(2):1099-106. doi: 10.4049/jimmunol.182.2.1099. J Immunol. 2009. PMID: 19124753
-
Self-Renewal and Toll-like Receptor Signaling Sustain Exhausted Plasmacytoid Dendritic Cells during Chronic Viral Infection.Immunity. 2018 Apr 17;48(4):730-744.e5. doi: 10.1016/j.immuni.2018.03.020. Immunity. 2018. PMID: 29669251 Free PMC article.
-
Plasmacytoid dendritic cells in antiviral immunity and autoimmunity.Sci China Life Sci. 2010 Feb;53(2):172-82. doi: 10.1007/s11427-010-0045-0. Epub 2010 Mar 7. Sci China Life Sci. 2010. PMID: 20596824 Free PMC article. Review.
-
Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9.Springer Semin Immunopathol. 2005 Jan;26(3):221-9. doi: 10.1007/s00281-004-0180-4. Epub 2004 Nov 13. Springer Semin Immunopathol. 2005. PMID: 15592841 Review.
Cited by
-
Immune response in SARS-CoV-2 infection: the role of interferons type I and type III.Braz J Infect Dis. 2020 Sep-Oct;24(5):428-433. doi: 10.1016/j.bjid.2020.07.011. Epub 2020 Aug 26. Braz J Infect Dis. 2020. PMID: 32866437 Free PMC article. Review.
-
An Overview of Current Knowledge of Deadly CoVs and Their Interface with Innate Immunity.Viruses. 2021 Mar 26;13(4):560. doi: 10.3390/v13040560. Viruses. 2021. PMID: 33810391 Free PMC article. Review.
-
N-acetyltransferase 10 regulates alphavirus replication via N4-acetylcytidine (ac4C) modification of the lymphocyte antigen six family member E (LY6E) mRNA.J Virol. 2024 Jan 23;98(1):e0135023. doi: 10.1128/jvi.01350-23. Epub 2024 Jan 3. J Virol. 2024. PMID: 38169284 Free PMC article.
-
Type I Interferon Signaling Disrupts the Hepatic Urea Cycle and Alters Systemic Metabolism to Suppress T Cell Function.Immunity. 2019 Dec 17;51(6):1074-1087.e9. doi: 10.1016/j.immuni.2019.10.014. Epub 2019 Nov 26. Immunity. 2019. PMID: 31784108 Free PMC article.
-
Immunometabolic Signature during Respiratory Viral Infection: A Potential Target for Host-Directed Therapies.Viruses. 2023 Feb 13;15(2):525. doi: 10.3390/v15020525. Viruses. 2023. PMID: 36851739 Free PMC article. Review.
References
-
- Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol. 2005;23:307–336. - PubMed
-
- Nguyen KB, Watford WT, Salomon R. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297:2063–2066. - PubMed
-
- Siegal FP, Kadowaki N, Shodell M. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. - PubMed
-
- Cella M, Jarrossay D, Facchetti F. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. - PubMed
Further Reading
-
- Adachi O, Kawai T, Takeda K. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

