p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness
- PMID: 16982802
- PMCID: PMC2064398
- DOI: 10.1083/jcb.200605022
p120 catenin is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness
Abstract
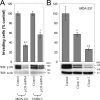
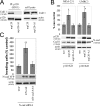
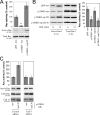
During epithelial tumor progression, the loss of E-cadherin expression and inappropriate expression of mesenchymal cadherins coincide with increased invasiveness. Reexpression experiments have established E-cadherin as an invasion suppressor. However, the mechanism by which E-cadherin suppresses invasiveness and the role of mesenchymal cadherins are poorly understood. We show that both p120 catenin and mesenchymal cadherins are required for the invasiveness of E-cadherin-deficient cells. p120 binding promotes the up-regulation of mesenchymal cadherins and the activation of Rac1, which are essential for cell migration and invasiveness. p120 also promotes invasiveness by inhibiting RhoA activity, independently of cadherin association. Furthermore, association of endogenous p120 with E-cadherin is required for E-cadherin-mediated suppression of invasiveness and is accompanied by a reduction in mesenchymal cadherin levels. The data indicate that p120 acts as a rheostat, promoting a sessile cellular phenotype when associated with E-cadherin or a motile phenotype when associated with mesenchymal cadherins.
Figures

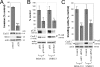
Similar articles
-
p120 catenin induces opposing effects on tumor cell growth depending on E-cadherin expression.J Cell Biol. 2008 Nov 17;183(4):737-49. doi: 10.1083/jcb.200805113. J Cell Biol. 2008. PMID: 19015320 Free PMC article.
-
Ablation of p120-catenin enhances invasion and metastasis of human lung cancer cells.Cancer Sci. 2009 Mar;100(3):441-8. doi: 10.1111/j.1349-7006.2008.01067.x. Epub 2008 Dec 22. Cancer Sci. 2009. PMID: 19154401 Free PMC article.
-
Inhibition of RhoA by p120 catenin.Nat Cell Biol. 2000 Sep;2(9):637-44. doi: 10.1038/35023588. Nat Cell Biol. 2000. PMID: 10980705
-
Protecting your tail: regulation of cadherin degradation by p120-catenin.Curr Opin Cell Biol. 2004 Oct;16(5):522-7. doi: 10.1016/j.ceb.2004.07.001. Curr Opin Cell Biol. 2004. PMID: 15363802 Review.
-
Regulation of cadherin stability and turnover by p120ctn: implications in disease and cancer.Semin Cell Dev Biol. 2004 Dec;15(6):657-63. doi: 10.1016/j.semcdb.2004.09.003. Semin Cell Dev Biol. 2004. PMID: 15561585 Review.
Cited by
-
XPC inhibits NSCLC cell proliferation and migration by enhancing E-Cadherin expression.Oncotarget. 2015 Apr 30;6(12):10060-72. doi: 10.18632/oncotarget.3542. Oncotarget. 2015. PMID: 25871391 Free PMC article.
-
Slug-upregulated miR-221 promotes breast cancer progression through suppressing E-cadherin expression.Sci Rep. 2016 May 13;6:25798. doi: 10.1038/srep25798. Sci Rep. 2016. PMID: 27174021 Free PMC article.
-
The Comparative Experimental Study of Sodium and Magnesium Dichloroacetate Effects on Pediatric PBT24 and SF8628 Cell Glioblastoma Tumors Using a Chicken Embryo Chorioallantoic Membrane Model and on Cells In Vitro.Int J Mol Sci. 2022 Sep 9;23(18):10455. doi: 10.3390/ijms231810455. Int J Mol Sci. 2022. PMID: 36142368 Free PMC article.
-
Upregulation of MUC5AC by VEGF in human primary bronchial epithelial cells: implications for asthma.Respir Res. 2019 Dec 12;20(1):282. doi: 10.1186/s12931-019-1245-1. Respir Res. 2019. PMID: 31831011 Free PMC article.
-
Cadherin and integrin regulation of epithelial cell migration.Langmuir. 2009 Sep 1;25(17):10092-9. doi: 10.1021/la901109e. Langmuir. 2009. PMID: 19583181 Free PMC article.
References
-
- Anastasiadis, P.Z., and A.B. Reynolds. 2001. Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13:604–610. - PubMed
-
- Anastasiadis, P.Z., S.Y. Moon, M.A. Thoreson, D.J. Mariner, H.C. Crawford, Y. Zheng, and A.B. Reynolds. 2000. Inhibition of RhoA by p120 catenin. Nat. Cell Biol. 2:637–644. - PubMed
-
- Bellovin, D.I., R.C. Bates, A. Muzikansky, D.L. Rimm, and A.M. Mercurio. 2005. Altered localization of p120 catenin during epithelial to mesenchymal transition of colon carcinoma is prognostic for aggressive disease. Cancer Res. 65:10938–10945. - PubMed
-
- Braga, V.M. 2002. Cell-cell adhesion and signalling. Curr. Opin. Cell Biol. 14:546–556. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

