Ptc1p regulates cortical ER inheritance via Slt2p
- PMID: 16977319
- PMCID: PMC1589985
- DOI: 10.1038/sj.emboj.7601319
Ptc1p regulates cortical ER inheritance via Slt2p
Abstract
Studies in the yeast Saccharomyces cerevisiae have shown that the inheritance of endoplasmic reticulum (ER), mitochondria, and vacuoles involves the capture of a tubular structure at the bud tip. Ptc1p, a serine/threonine phosphatase, has previously been shown to regulate mitochondrial inheritance by an unknown mechanism. Ptc1p regulates the high osmolarity glycerol mitogen-activated protein kinase (MAPK) pathway and has also been implicated in the cell wall integrity (CWI) MAPK pathway. Here we show that the loss of Ptc1p or the Ptc1p binding protein, Nbp2p, causes a prominent delay in the delivery of ER tubules to the periphery of daughter cells and results in a dramatic increase in the level of phosphorylated Slt2p, the MAPK in the CWI pathway. Either loss of Slt2p or inhibition of the CWI pathway by addition of sorbitol, suppresses the ER inheritance defect in the ptc1Delta and nbp2Delta mutants. Our findings indicate that Ptc1p and Nbp2p regulate ER inheritance through the CWI MAPK pathway by modulating the MAPK, Slt2p.
Figures
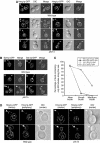
 ), 253 wild-type buds expressing Ssh1p-GFP (–•–), 264 ptc1Δ buds expressing Hmg1p-GFP (
), 253 wild-type buds expressing Ssh1p-GFP (–•–), 264 ptc1Δ buds expressing Hmg1p-GFP ( ), and 249 ptc1Δ buds expressing Ssh1p-GFP (–○–) was quantitated. The cortical ER tubular network is normal in ptc1Δ mother cells (D). A stack of images of wild-type (SFNY1625) and ptc1Δ (SFNY1624) diploid cells with focal planes 0.1-μm apart were obtained and deconvolved. Images obtained from the center of the cell and from the cell periphery are shown. Arrowheads point to medium buds and arrows point to large buds. Bars, 5 μm.
), and 249 ptc1Δ buds expressing Ssh1p-GFP (–○–) was quantitated. The cortical ER tubular network is normal in ptc1Δ mother cells (D). A stack of images of wild-type (SFNY1625) and ptc1Δ (SFNY1624) diploid cells with focal planes 0.1-μm apart were obtained and deconvolved. Images obtained from the center of the cell and from the cell periphery are shown. Arrowheads point to medium buds and arrows point to large buds. Bars, 5 μm.



 ), 253 nbp2Δ buds expressing Hmg1p-GFP (–▵–), 234 wild-type buds expressing Ssh1p-GFP (
), 253 nbp2Δ buds expressing Hmg1p-GFP (–▵–), 234 wild-type buds expressing Ssh1p-GFP ( ), and 260 nbp2Δ buds expressing Ssh1p-GFP (
), and 260 nbp2Δ buds expressing Ssh1p-GFP ( ).Bars, 5 μm.
).Bars, 5 μm.
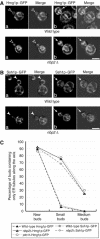
 ), 139 ptc1Δ hog1Δ buds (–○–), 278 wild-type buds cultured in SC+1 M sorbitol (–⧫–), 275 ptc1Δ buds grown in SC medium (
), 139 ptc1Δ hog1Δ buds (–○–), 278 wild-type buds cultured in SC+1 M sorbitol (–⧫–), 275 ptc1Δ buds grown in SC medium ( ), 284 ptc1Δ buds grown in SC+1 M sorbitol (
), 284 ptc1Δ buds grown in SC+1 M sorbitol ( ), 296 nbp2Δ buds grown in SC medium (–▵–), 284 nbp2Δ buds grown in SC+1 M sorbitol (–▴–) were analyzed. (F) Deletion of slt2 had no effect on vacuole distribution in ptc1Δ mutant and wild-type cells. The vacuole inheritance defect was analyzed as in Figure 3B. Bars, 5 μm.
), 296 nbp2Δ buds grown in SC medium (–▵–), 284 nbp2Δ buds grown in SC+1 M sorbitol (–▴–) were analyzed. (F) Deletion of slt2 had no effect on vacuole distribution in ptc1Δ mutant and wild-type cells. The vacuole inheritance defect was analyzed as in Figure 3B. Bars, 5 μm.
Similar articles
-
Yeast adaptor protein, Nbp2p, is conserved regulator of fungal Ptc1p phosphatases and is involved in multiple signaling pathways.J Biol Chem. 2012 Jun 22;287(26):22133-41. doi: 10.1074/jbc.M112.348052. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570491 Free PMC article.
-
Different polarisome components play distinct roles in Slt2p-regulated cortical ER inheritance in Saccharomyces cerevisiae.Mol Biol Cell. 2013 Oct;24(19):3145-54. doi: 10.1091/mbc.E13-05-0268. Epub 2013 Aug 7. Mol Biol Cell. 2013. PMID: 23924898 Free PMC article.
-
Activation of the mitogen-activated protein kinase, Slt2p, at bud tips blocks a late stage of endoplasmic reticulum inheritance in Saccharomyces cerevisiae.Mol Biol Cell. 2010 May 15;21(10):1772-82. doi: 10.1091/mbc.e09-06-0532. Epub 2010 Mar 31. Mol Biol Cell. 2010. PMID: 20357006 Free PMC article.
-
Control of Gene Expression via the Yeast CWI Pathway.Int J Mol Sci. 2022 Feb 4;23(3):1791. doi: 10.3390/ijms23031791. Int J Mol Sci. 2022. PMID: 35163713 Free PMC article. Review.
-
Protein phosphatases in MAPK signalling: we keep learning from yeast.Mol Microbiol. 2005 Oct;58(1):6-16. doi: 10.1111/j.1365-2958.2005.04822.x. Mol Microbiol. 2005. PMID: 16164545 Review.
Cited by
-
A protein complex containing Epo1p anchors the cortical endoplasmic reticulum to the yeast bud tip.J Cell Biol. 2015 Jan 5;208(1):71-87. doi: 10.1083/jcb.201407126. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547157 Free PMC article.
-
A Conserved residue in the yeast Bem1p SH3 domain maintains the high level of binding specificity required for function.J Biol Chem. 2011 Jun 3;286(22):19470-7. doi: 10.1074/jbc.M111.229294. Epub 2011 Apr 12. J Biol Chem. 2011. PMID: 21489982 Free PMC article.
-
Yeast adaptor protein, Nbp2p, is conserved regulator of fungal Ptc1p phosphatases and is involved in multiple signaling pathways.J Biol Chem. 2012 Jun 22;287(26):22133-41. doi: 10.1074/jbc.M112.348052. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570491 Free PMC article.
-
Different polarisome components play distinct roles in Slt2p-regulated cortical ER inheritance in Saccharomyces cerevisiae.Mol Biol Cell. 2013 Oct;24(19):3145-54. doi: 10.1091/mbc.E13-05-0268. Epub 2013 Aug 7. Mol Biol Cell. 2013. PMID: 23924898 Free PMC article.
-
Sticking With It: ER-PM Membrane Contact Sites as a Coordinating Nexus for Regulating Lipids and Proteins at the Cell Cortex.Front Cell Dev Biol. 2020 Jul 22;8:675. doi: 10.3389/fcell.2020.00675. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32793605 Free PMC article. Review.
References
-
- Acharya U, Mallabiabarrena A, Acharya JK, Malhotra V (1998) Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92: 183–192 - PubMed
-
- Andrews PD, Stark MJ (2000) Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J Cell Sci 113: 2685–2693 - PubMed
-
- Boldogh IR, Fehrenbacher KL, Yang HC, Pon LA (2005) Mitochondrial movement and inheritance in budding yeast. Gene 354: 28–36 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

