Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion
- PMID: 16973550
- PMCID: PMC1617263
- DOI: 10.1128/JVI.00572-06
Soluble Epstein-Barr virus glycoproteins gH, gL, and gp42 form a 1:1:1 stable complex that acts like soluble gp42 in B-cell fusion but not in epithelial cell fusion
Abstract
Epstein-Barr virus (EBV) is a herpesvirus that infects cells by fusing its lipid envelope with the target cell membrane. The fusion process requires the actions of viral glycoproteins gH, gL, and gB for entry into epithelial cells and additionally requires gp42 for entry into B cells. To further study the roles of these membrane-associated glycoproteins, purified soluble forms of gp42, gH, and gL were expressed that lack the membrane-spanning regions. The soluble gH/gL protein complex binds to soluble gp42 with high affinity, forming a stable heterotrimer with 1:1:1 stoichiometry, and this complex is not formed by an N-terminally truncated variant of gp42. The effects of adding soluble gp42, gH/gL, and gH/gL/gp42 were examined with a virus-free cell-cell fusion assay. The results demonstrate that, in contrast to gp42, membrane fusion does not proceed with secreted gH/gL. The addition of soluble gH/gL does not inhibit or enhance B-cell or epithelial cell fusion when membrane-bound gH/gL, gB, and gp42 are present. However, the soluble gH/gL/gp42 complex does activate membrane fusion with B cells, similarly to soluble gp42, but it does not inhibit fusion with epithelial cells, as observed for gp42 alone. A gp42 peptide, derived from an N-terminal segment involved in gH/gL interactions, binds to soluble gH/gL and inhibits EBV-mediated epithelial cell fusion, mimicking gp42. These observations reveal distinct functional requirements for gH/gL and gp42 complexes in EBV-mediated membrane fusion.
Figures
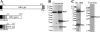

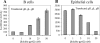
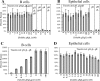
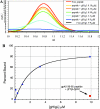
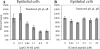
Similar articles
-
Binding-site interactions between Epstein-Barr virus fusion proteins gp42 and gH/gL reveal a peptide that inhibits both epithelial and B-cell membrane fusion.J Virol. 2007 Sep;81(17):9216-29. doi: 10.1128/JVI.00575-07. Epub 2007 Jun 20. J Virol. 2007. PMID: 17581996 Free PMC article.
-
Functional homology of gHs and gLs from EBV-related gamma-herpesviruses for EBV-induced membrane fusion.Virology. 2007 Aug 15;365(1):157-65. doi: 10.1016/j.virol.2007.03.054. Epub 2007 May 2. Virology. 2007. PMID: 17477951 Free PMC article.
-
Membrane anchoring of Epstein-Barr virus gp42 inhibits fusion with B cells even with increased flexibility allowed by engineered spacers.mBio. 2015 Jan 6;6(1):e02285-14. doi: 10.1128/mBio.02285-14. mBio. 2015. PMID: 25564465 Free PMC article.
-
Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus.Mol Cells. 2016 Apr 30;39(4):286-91. doi: 10.14348/molcells.2016.0066. Epub 2016 Apr 6. Mol Cells. 2016. PMID: 27094060 Free PMC article. Review.
-
[The entry of Epstein-Barr virus into B lymphocytes and epithelial cells during infection].Bing Du Xue Bao. 2014 Jul;30(4):476-82. Bing Du Xue Bao. 2014. PMID: 25272606 Review. Chinese.
Cited by
-
Herpes virus fusion and entry: a story with many characters.Viruses. 2012 May;4(5):800-32. doi: 10.3390/v4050800. Epub 2012 May 10. Viruses. 2012. PMID: 22754650 Free PMC article. Review.
-
Epstein-Barr Virus and the Human Leukocyte Antigen Complex.Curr Clin Microbiol Rep. 2019 Sep;6(3):175-181. doi: 10.1007/s40588-019-00120-9. Epub 2019 Jul 8. Curr Clin Microbiol Rep. 2019. PMID: 33094090 Free PMC article.
-
Virus-Induced Cell Fusion and Syncytia Formation.Results Probl Cell Differ. 2024;71:283-318. doi: 10.1007/978-3-031-37936-9_14. Results Probl Cell Differ. 2024. PMID: 37996683
-
Hydrophobic residues that form putative fusion loops of Epstein-Barr virus glycoprotein B are critical for fusion activity.J Virol. 2007 Sep;81(17):9596-600. doi: 10.1128/JVI.00758-07. Epub 2007 Jun 6. J Virol. 2007. PMID: 17553877 Free PMC article.
-
Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells.J Virol. 2008 Jan;82(1):60-70. doi: 10.1128/JVI.01910-07. Epub 2007 Oct 17. J Virol. 2008. PMID: 17942555 Free PMC article.
References
-
- Amon, W., and P. J. Farrell. 2005. Reactivation of Epstein-Barr virus from latency. Rev. Med. Virol. 15:149-156. - PubMed
-
- Borza, C. M., and L. Hutt-Fletcher. 2002. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 8:594-599. - PubMed
-
- Gasteiger, E., C. Hoogland, A. Gattiker, S. Duvaud, M. R. Wilkins, R. D. Appel, and A. Bairoch. 2005. Protein identification and analysis tools on the ExPASy server, p. 571-607. In J. M. Walker (ed.), The proteomics protocols handbook. Humana Press, Totowa, N.J.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

