Solution structure of the complex between poxvirus-encoded CC chemokine inhibitor vCCI and human MIP-1beta
- PMID: 16963564
- PMCID: PMC1599900
- DOI: 10.1073/pnas.0602142103
Solution structure of the complex between poxvirus-encoded CC chemokine inhibitor vCCI and human MIP-1beta
Abstract
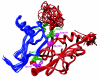
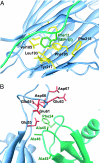
Chemokines (chemotactic cytokines) comprise a large family of proteins that recruit and activate leukocytes, giving chemokines a major role in both immune response and inflammation-related diseases. The poxvirus-encoded viral CC chemokine inhibitor (vCCI) binds to many CC chemokines with high affinity, acting as a potent inhibitor of chemokine action. We have used heteronuclear multidimensional NMR to determine the structure of an orthopoxvirus vCCI in complex with a human CC chemokine, MIP-1beta (macrophage inflammatory protein 1beta). vCCI binds to the chemokine with 1:1 stoichiometry, forming a complex of 311 aa. vCCI uses residues from its beta-sheet II to interact with a surface of MIP-1beta that includes residues adjacent to its N terminus, as well as residues in the 20's region and the 40's loop. This structure reveals the strategy used by vCCI to tightly bind numerous chemokines while retaining selectivity for the CC chemokine subfamily.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



Similar articles
-
Structural insights into the interaction between a potent anti-inflammatory protein, viral CC chemokine inhibitor (vCCI), and the human CC chemokine, Eotaxin-1.J Biol Chem. 2014 Mar 7;289(10):6592-6603. doi: 10.1074/jbc.M113.538991. Epub 2014 Jan 30. J Biol Chem. 2014. PMID: 24482230 Free PMC article.
-
Biophysical and Computational Studies of the vCCI:vMIP-II Complex.Int J Mol Sci. 2017 Aug 16;18(8):1778. doi: 10.3390/ijms18081778. Int J Mol Sci. 2017. PMID: 28813018 Free PMC article.
-
Comprehensive mapping of poxvirus vCCI chemokine-binding protein. Expanded range of ligand interactions and unusual dissociation kinetics.J Biol Chem. 2002 Jan 25;277(4):2785-9. doi: 10.1074/jbc.M109884200. Epub 2001 Nov 5. J Biol Chem. 2002. PMID: 11696549
-
The binding and specificity of chemokine binding proteins, through the lens of experiment and computation.Biomed J. 2022 Jun;45(3):439-453. doi: 10.1016/j.bj.2021.07.004. Epub 2021 Jul 24. Biomed J. 2022. PMID: 34311129 Free PMC article. Review.
-
Viral chemokine-binding proteins.J Leukoc Biol. 2002 Jul;72(1):24-34. J Leukoc Biol. 2002. PMID: 12101259 Review.
Cited by
-
The Role of Chemokines in Wound Healing.Int J Mol Sci. 2018 Oct 18;19(10):3217. doi: 10.3390/ijms19103217. Int J Mol Sci. 2018. PMID: 30340330 Free PMC article. Review.
-
Mechanism of action of the viral chemokine-binding protein E163 from ectromelia virus.J Biol Chem. 2018 Nov 9;293(45):17418-17429. doi: 10.1074/jbc.RA118.004432. Epub 2018 Sep 26. J Biol Chem. 2018. PMID: 30257868 Free PMC article.
-
Structure and function of A41, a vaccinia virus chemokine binding protein.PLoS Pathog. 2008 Jan;4(1):e5. doi: 10.1371/journal.ppat.0040005. PLoS Pathog. 2008. PMID: 18208323 Free PMC article.
-
A 3D structural SARS-CoV-2-human interactome to explore genetic and drug perturbations.Nat Methods. 2021 Dec;18(12):1477-1488. doi: 10.1038/s41592-021-01318-w. Epub 2021 Nov 29. Nat Methods. 2021. PMID: 34845387 Free PMC article.
-
Site-directed mutagenesis of the CC chemokine binding protein 35K-Fc reveals residues essential for activity and mutations that increase the potency of CC chemokine blockade.Mol Pharmacol. 2011 Aug;80(2):328-36. doi: 10.1124/mol.111.071985. Epub 2011 May 17. Mol Pharmacol. 2011. PMID: 21586597 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

