Cytomegalovirus-induced embryopathology: mouse submandibular salivary gland epithelial-mesenchymal ontogeny as a model
- PMID: 16959038
- PMCID: PMC1601957
- DOI: 10.1186/1471-213X-6-42
Cytomegalovirus-induced embryopathology: mouse submandibular salivary gland epithelial-mesenchymal ontogeny as a model
Abstract
Background: Human studies suggest, and mouse models clearly demonstrate, that cytomegalovirus (CMV) is dysmorphic to early organ and tissue development. CMV has a particular tropism for embryonic salivary gland and other head mesenchyme. CMV has evolved to co-opt cell signaling networks so to optimize replication and survival, to the detriment of infected tissues. It has been postulated that mesenchymal infection is the critical step in disrupting organogenesis. If so, organogenesis dependent on epithelial-mesenchymal interactions would be particularly vulnerable. In this study, we chose to model the vulnerability by investigating the cell and molecular pathogenesis of CMV infected mouse embryonic submandibular salivary glands (SMGs).
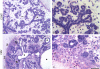
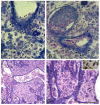
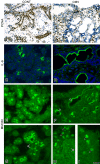
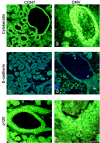
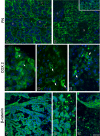
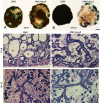
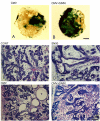
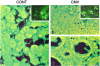
Results: We infected E15 SMG explants with mouse CMV (mCMV). Active infection for up to 12 days in vitro results in a remarkable cell and molecular pathology characterized by atypical ductal epithelial hyperplasia, apparent epitheliomesenchymal transformation, oncocytic-like stromal metaplasia, beta-catenin nuclear localization, and upregulation of Nfkb2, Relb, Il6, Stat3, and Cox2. Rescue with an antiviral nucleoside analogue indicates that mCMV replication is necessary to initiate and maintain SMG dysmorphogenesis.
Conclusion: mCMV infection of embryonic mouse explants results in dysplasia, metaplasia, and, possibly, anaplasia. The molecular pathogenesis appears to center around the activation of canonical and, perhaps more importantly, noncanonical NFkappaB. Further, COX-2 and IL-6 are important downstream effectors of embryopathology. At the cellular level, there appears to be a consequential interplay between the transformed SMG cells and the surrounding extracellular matrix, resulting in the nuclear translocation of beta-catenin. From these studies, a tentative framework has emerged within which additional studies may be planned and performed.
Figures



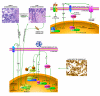
 single or multistep stimulatory modification of unknown mechanism. ⊣ single or multistep inhibitory modification. RKT: Receptor Tyrosine Kinase. DNA: host DNA. vDNA: viral DNA.
single or multistep stimulatory modification of unknown mechanism. ⊣ single or multistep inhibitory modification. RKT: Receptor Tyrosine Kinase. DNA: host DNA. vDNA: viral DNA.Similar articles
-
Cytomegalovirus induces abnormal chondrogenesis and osteogenesis during embryonic mandibular development.BMC Dev Biol. 2008 Mar 27;8:33. doi: 10.1186/1471-213X-8-33. BMC Dev Biol. 2008. PMID: 18371224 Free PMC article.
-
Salivary glands and human congenital cytomegalovirus infection: What happens in early fetal life?J Med Virol. 2017 Feb;89(2):318-323. doi: 10.1002/jmv.24628. Epub 2016 Jul 25. J Med Virol. 2017. PMID: 27420192
-
Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis.Dev Dyn. 2004 Apr;229(4):722-32. doi: 10.1002/dvdy.10472. Dev Dyn. 2004. PMID: 15042696
-
Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection.Pathol Int. 1995 Feb;45(2):91-102. doi: 10.1111/j.1440-1827.1995.tb03428.x. Pathol Int. 1995. PMID: 7742931 Review.
-
Salivary gland organogenesis.Wiley Interdiscip Rev Dev Biol. 2012 Jan-Feb;1(1):69-82. doi: 10.1002/wdev.4. Epub 2011 Nov 17. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801668 Review.
Cited by
-
Later passages of neural progenitor cells from neonatal brain are more permissive for human cytomegalovirus infection.J Virol. 2013 Oct;87(20):10968-79. doi: 10.1128/JVI.01120-13. Epub 2013 Jul 31. J Virol. 2013. PMID: 23903847 Free PMC article.
-
CRTC1 expression during normal and abnormal salivary gland development supports a precursor cell origin for mucoepidermoid cancer.Gene Expr Patterns. 2011 Jan-Feb;11(1-2):57-63. doi: 10.1016/j.gep.2010.09.003. Epub 2010 Sep 15. Gene Expr Patterns. 2011. PMID: 20837164 Free PMC article.
-
Environmental mechanisms of orofacial clefts.Birth Defects Res. 2020 Nov;112(19):1660-1698. doi: 10.1002/bdr2.1830. Epub 2020 Oct 30. Birth Defects Res. 2020. PMID: 33125192 Free PMC article. Review.
-
Constitutive β-catenin signaling by the viral chemokine receptor US28.PLoS One. 2012;7(11):e48935. doi: 10.1371/journal.pone.0048935. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23145028 Free PMC article.
-
Cytomegalovirus inhibition of embryonic mouse tooth development: a model of the human amelogenesis imperfecta phenocopy.Arch Oral Biol. 2008 May;53(5):405-15. doi: 10.1016/j.archoralbio.2007.11.014. Epub 2008 Jan 16. Arch Oral Biol. 2008. PMID: 18201685 Free PMC article.
References
-
- Smith MG Propagation in tissue cultures of a cytopathogenic virus from human salivary gland virus (SGV) disease. Proc Soc Exp Biol Med. 1956;92:424–430. - PubMed
-
- Weller TH The cytomegaloviruses: Ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971;285:203–214. - PubMed
-
- Weller TH The cytomegaloviruses: Ubiquitous agents with protean clinical manifestations. II. N Engl J Med. 1971;285:267–274. - PubMed
-
- Davison AJ . Comparative betaherpesvirus genome and virion structure. In: Arvin AM, Mocarski ES, Moore P, Whitley R, Yamanishi K, Gampadelli-Fiume G and Roizman B, editor. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge, Cambridge Press; 2006. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

